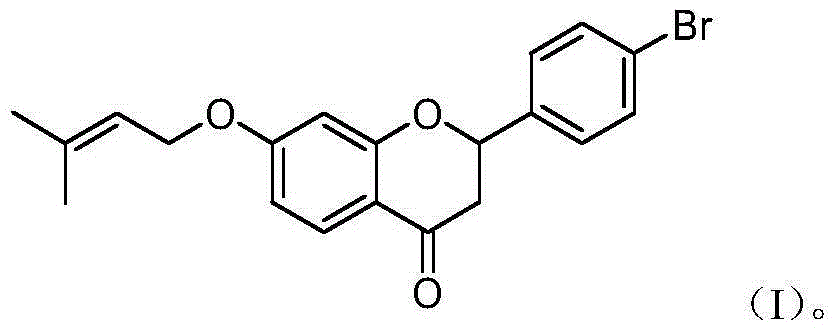
4'-bromo-7-isoamylene oxo-2,3-flavanone and preparation method as well as application in preparing antidepressant drugs
A technology of prenyloxy and dihydroflavone, which is applied in the direction of drug combinations, pharmaceutical formulas, and medical preparations containing active ingredients, etc., can solve the problems of side effects and discomfort, and limit long-term use, etc., to achieve rapid onset of action , low toxicity and side effects, good safety effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 14
[0023] The preparation of embodiment 14'-bromo-7-prenyloxy-2,3-dihydroflavone
[0024] Take 0.50 g of 4 mmol of compound II (2-hydroxyl-4-prenyloxyacetophenone), add p-bromobenzaldehyde (8 mmol) to compound II, and add dropwise 4.89 g of KOH under the protection of nitrogen flow. 14% KOH solution prepared with 43.60ml of absolute ethanol, reacted at room temperature for 8h. TLC followed the reaction until the raw material point disappeared, and the reaction was stopped. The reaction solution was poured into ice water, adjusted to neutral pH with HCl, and extracted with ether. The ether layers were combined, the ether was distilled off under reduced pressure, the silica gel column with a specification of 300-400 mesh was used to lay the column, and the column was passed through petroleum ether / ethyl acetate [V (petroleum ether) / V (ethyl acetate)] = 20:1 to obtain Yellow needle-like crystal 4′-bromo-2-hydroxy-4-prenyloxychalcone; take 0.50g of 4′-bromo-2-hydroxy-4-prenyloxycha...
Embodiment 2
[0028] Embodiment 2 antidepressant pharmacological experiment and neurotoxicity experiment
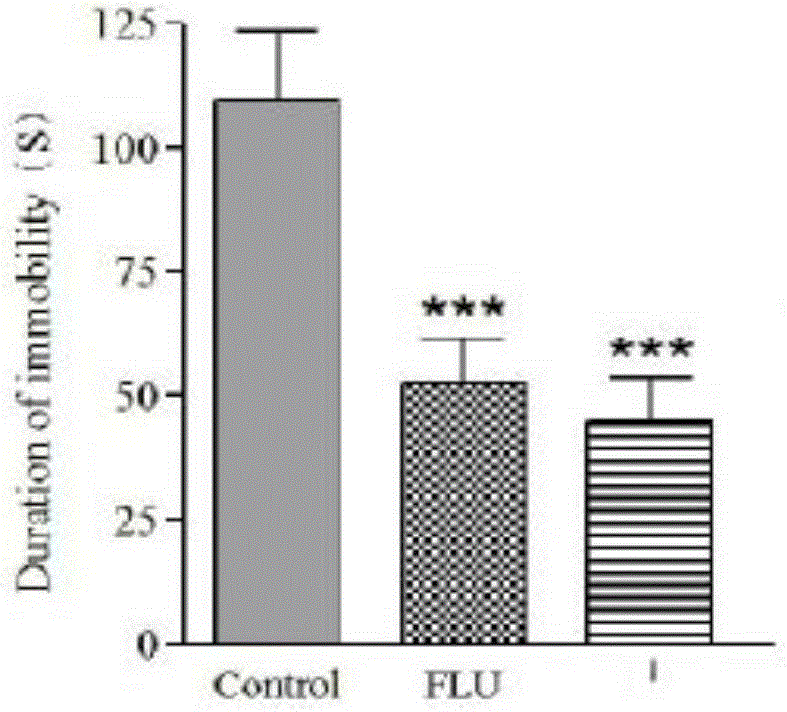
[0029] The forced swimming test and the tail suspension test are the two most commonly used experiments for depressant and antidepressant research. The sum of the time for mice to give up swimming and struggling in the water and to give up struggling when suspended in mid-air is called amobility The time reflects the symptoms of helplessness in mice, while the extension of immobility time in the forced swimming test and tail suspension test shows depression-like symptoms. The compound provided by the invention is screened by the mouse forced swimming test and the mouse tail spin test to evaluate its antidepressant efficacy and safety.
[0030] (1) Mice forced swimming test:
[0031]a method: see (PorsoltRDetal, ArchIntPharmaeodynTher, 1997) and other reported methods, after intraperitoneal injection for 30 minutes, put the mouse into the XSC-mouse constant temperature swimming instrum...
Embodiment 3
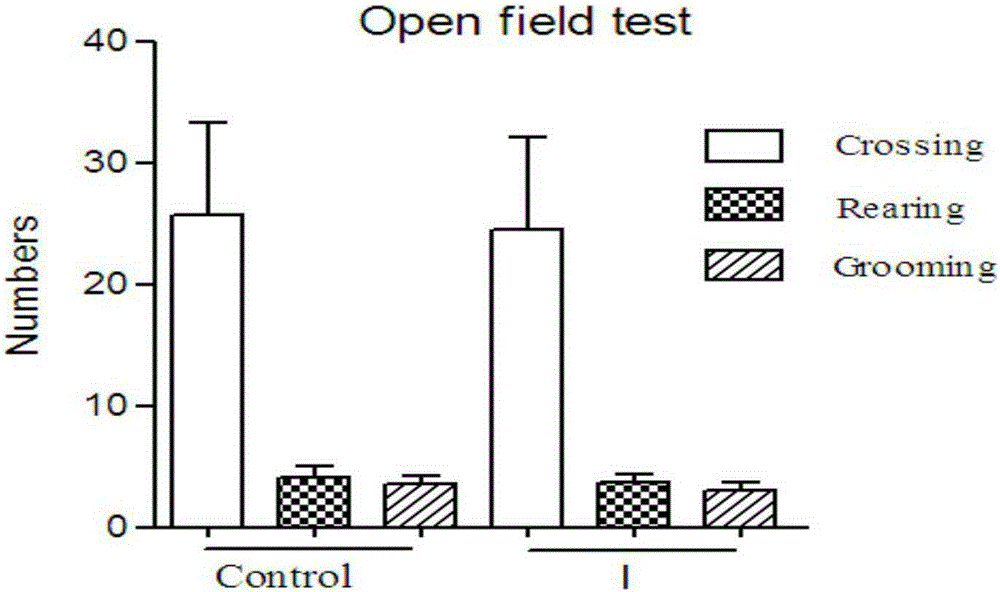
[0050] Embodiment 3 open experiment autonomous activity
[0051] The open spontaneous activity test is mainly used to evaluate the autonomous activity behavior of experimental animals. It is a common method for central nervous system safety pharmacology evaluation and neuroscience research. Important methodological experiments that provide parameters for scientific research purposes.
[0052] Method a: In an opaque plastic box (80cm×60cm×30cm), the bottom of the plastic box is divided into 48 square grids with a size of 10cm×10cm. Place the plastic box in a quiet room and light it with a 60W light bulb, which is about 1m above the center of the plastic box.
[0053] b Grouping: 20 male mice were randomly divided into two groups, 10 in each group, which were blank control group and drug group respectively. 30 minutes before the test, compound I group: dissolved in DMSO solution, intraperitoneally injected 0.10ml / 20g at a dose of 10mg / kg, and the blank control group was intrap...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com