Lactose celecoxib pharmaceutical composition
A technology of celecoxib and composition, applied in the pharmaceutical field, can solve problems such as high elasticity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
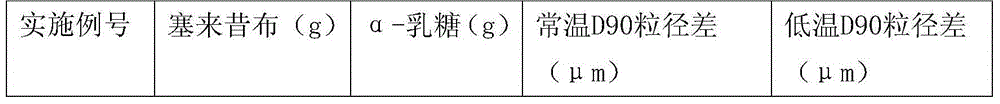
[0031] Dissolve the solid α-lactose monohydrate and celecoxib completely in 55% ethanol aqueous solution (55% of the volume ratio of ethanol) at 60°C, and distill under reduced pressure until the ethanol content in the ethanol aqueous solution is 30-35%, -10 to - Freeze at 5°C, filter, and dry the solid in a vacuum oven under reduced pressure. The ratio of α-lactose and celecoxib is shown in the table below. The α-lactose and celecoxib solid dispersions are simply pulverized and passed through the No. 2 sieve, and the D90 particle size is measured by a laser particle size analyzer, and then pulverized twice by a jet mill, the pulverization time is 10 minutes / time, and then used The D90 particle size of the powder is measured by the laser particle size analyzer, and the difference between the two is obtained by subtracting the D90 particle size.
[0032]
[0033]
[0034] Examples 1-1 to 1-5 have melting points between 166-173°C.
Embodiment 2
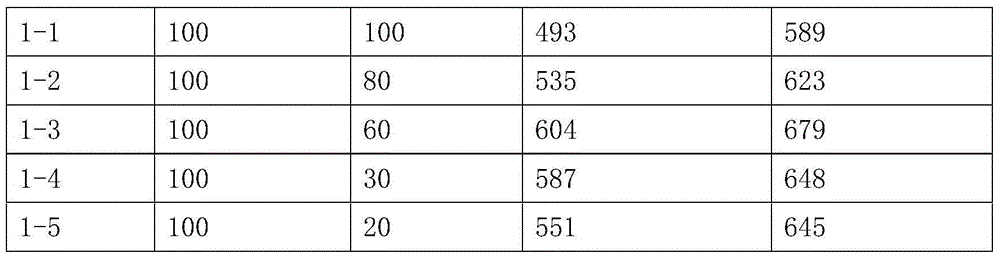
[0036] Dissolve the solid α-lactose monohydrate and celecoxib completely in 55% ethanol aqueous solution (55% of the volume ratio of ethanol) at 60°C, and distill under reduced pressure until the ethanol content in the ethanol aqueous solution is 30-35%, -10 to - Freeze at 5°C, filter, and dry the solid in a vacuum oven under reduced pressure. The ratio of α-lactose and celecoxib is shown in the table below. The solid dispersion of sodium lauryl sulfate, α-lactose, and celecoxib is passed through No. 2 sieve after being simply pulverized, and the D90 particle size is measured with a laser particle size analyzer, and then pulverized twice by a jet mill, and the pulverization time is 10 minutes / time, and then use a laser particle size analyzer to measure the D90 particle size of the powder, and subtract the two to get the D90 particle size difference. .
[0037]
[0038] Examples 2-1 to 2-5 have melting points between 161-170°C.
Embodiment 3
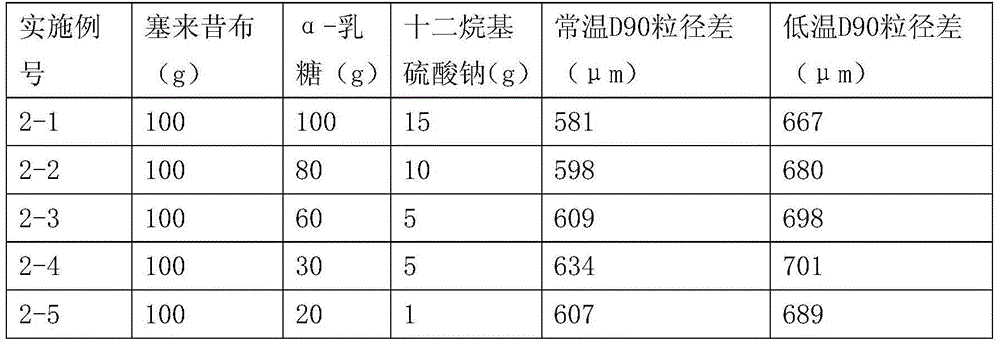
[0040] Dissolve the solid α-lactose monohydrate and celecoxib completely in 55% ethanol aqueous solution (55% by volume of ethanol) at 60°C, distill under reduced pressure until the ethanol content in the ethanol aqueous solution is 30-35%, -10 to - Freeze at 5°C, filter, and dry the solid in a vacuum oven under reduced pressure. The ratio of α-lactose and celecoxib is shown in the table below. The α-lactose and celecoxib solid dispersions are simply pulverized and passed through the No. 2 sieve, and the D90 particle size is measured by a laser particle size analyzer, and then pulverized twice by a jet mill, the pulverization time is 10 minutes / time, and then used The D90 particle size of the powder is measured by the laser particle size analyzer, and the difference between the two is subtracted to obtain the D90 particle size difference.
[0041]
[0042]
[0043] Examples 3-1 to 3-5 have melting points between 170-178°C.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com