Synthesis and refining method of thiamethoxam intermediate 2-chloroallyl isothiocyanate
A technology of allyl thiocyanate and chloroisothiocyanate, which is applied in the field of synthesis and purification of thiamethoxam intermediate 2-chloroisothiocyanate, and can solve problems such as complicated operation, low yield and the like , to achieve the effect of simplifying the operation process, good yield and reducing cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
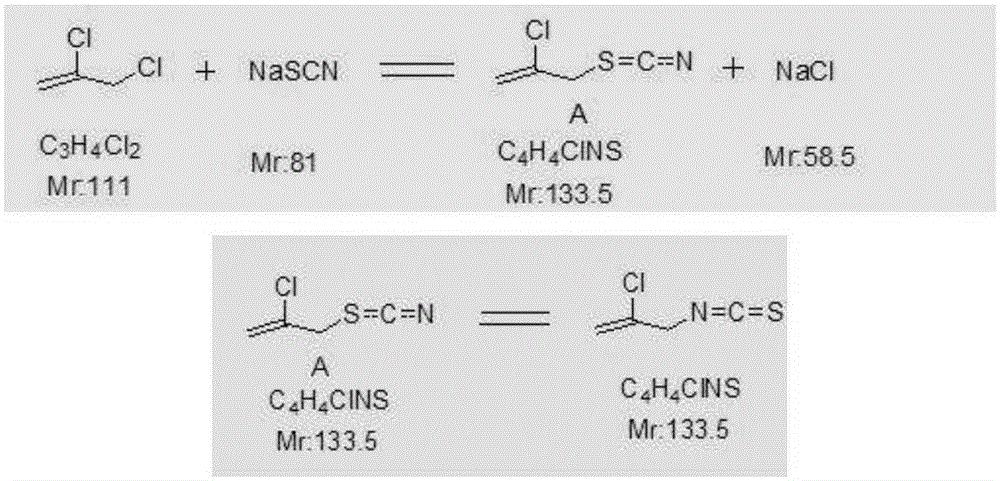
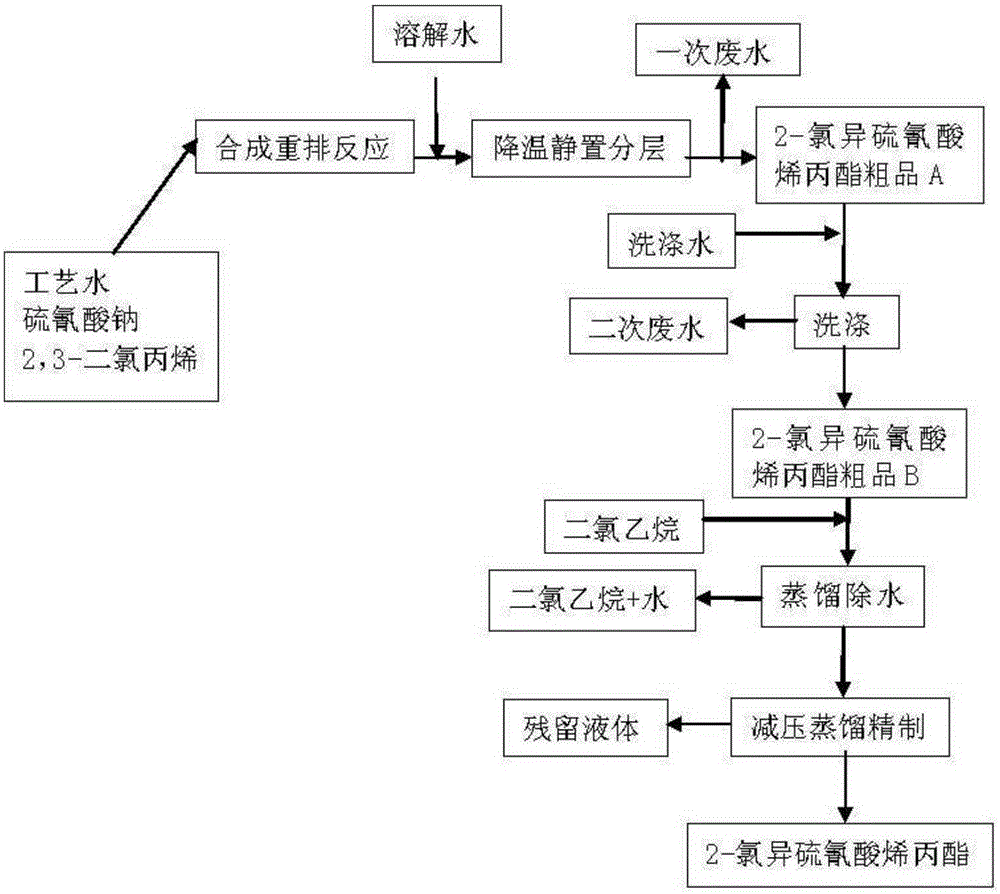
[0025] 1) Synthesis: Add 410g of process water, 293g of solid sodium thiocyanate and 370g of 2,3-dichloropropene into the reactor, stir, heat up to 84°C for reflux, and reflux for 12 hours. During the reaction, the temperature gradually rises to 112°C about. After the reaction finishes, add 250g dissolved water, the solid salt that generates is dissolved completely, and material temperature is cooled to 40 ℃, carry out static layering, separate 836g primary waste water and 478g2-allyl chloroisothiocyanate crude product A, The content is about 89%, and the crude yield is about 95.6%.
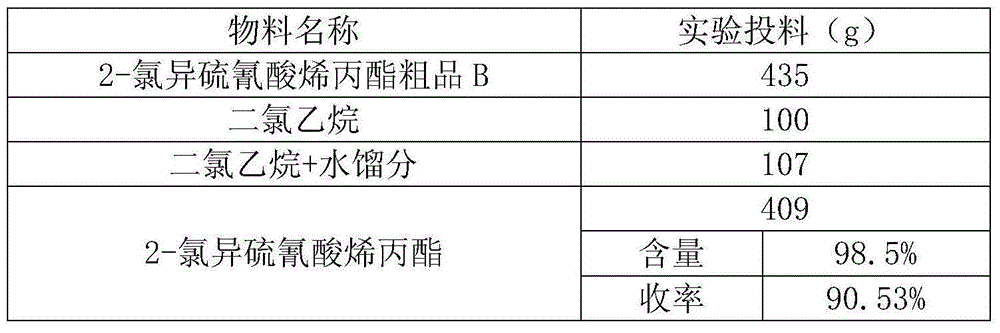
[0026] The experimental data of this synthesis step is shown in Table 1-1:
[0027] Table 1-1 Experimental data table
[0028] Material name Experiment feeding (g) 2,3-Dichloropropene 370 Sodium thiocyanate 293 process water 410 dissolved water 250
[0029] primary waste water 836 Allyl 2-Chloroisothiocyanate Crude A 478
[0030] 2)...
Embodiment 2
[0040] (1) With 2,3-dichloropropene and sodium thiocyanate as the reaction raw materials, in the presence of water, thiocyanate replaces chloride ions to generate allyl thiocyanate, and then regenerates from allyl thiocyanate Row to generate allyl 2-chloroisothiocyanate; the mass position of 2,3-dichloropropene and process water is 1:0.7; the molar ratio of 2,3-dichloropropene to sodium thiocyanate is 1:0.95 ; Reaction temperature is 50°C; Reaction time is 1 hour
[0041] (2) After the synthesis reaction is finished, the material is cooled, allowed to stand, and layered to obtain the crude product of allyl 2-chloroisothiocyanate;
[0042] (3) The crude product of allyl 2-chloroisothiocyanate obtained in step (2) is treated and refined through water removal to obtain the allyl 2-chloroisothiocyanate product; wherein the water removal process uses two Ethyl chloride and water are azeotroped to remove water. The mass ratio of dichloroethane and crude allyl 2-chloroisothiocyanate...
Embodiment 3
[0047](1) With 2,3-dichloropropene and sodium thiocyanate as the reaction raw materials, in the presence of water, thiocyanate replaces chloride ions to generate allyl thiocyanate, and then regenerates from allyl thiocyanate Row to generate allyl 2-chloroisothiocyanate; the mass position of 2,3-dichloropropene and water is 1:1.25; the molar ratio of 2,3-dichloropropene to sodium thiocyanate is 1:1.1; The reaction temperature is 120°C; the reaction time is 24 hours
[0048] (2) After the synthesis reaction is finished, the material is cooled, allowed to stand, and layered to obtain the crude product of allyl 2-chloroisothiocyanate;
[0049] (3) The crude product of allyl 2-chloroisothiocyanate obtained in step (2) is treated and refined through water removal to obtain the allyl 2-chloroisothiocyanate product; wherein the water removal process uses two Ethyl chloride and water are azeotroped to remove water. The mass ratio of dichloroethane and crude allyl 2-chloroisothiocyanat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com