Enrofloxacin injection and preparation method thereof
A technology of enrofloxacin and injection, which is used in pharmaceutical formulations, medical preparations with non-active ingredients, and medical preparations containing active ingredients, etc. problem, to achieve the effect of promoting dissolution and reducing pH value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
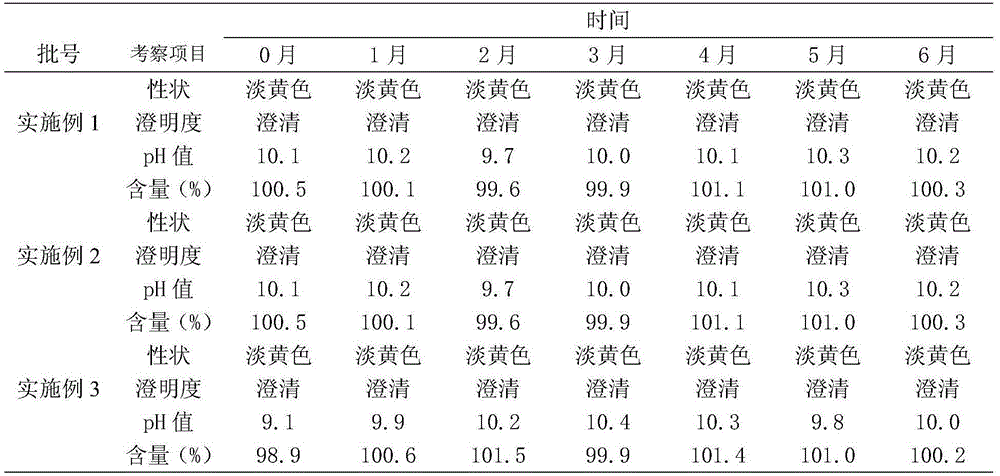
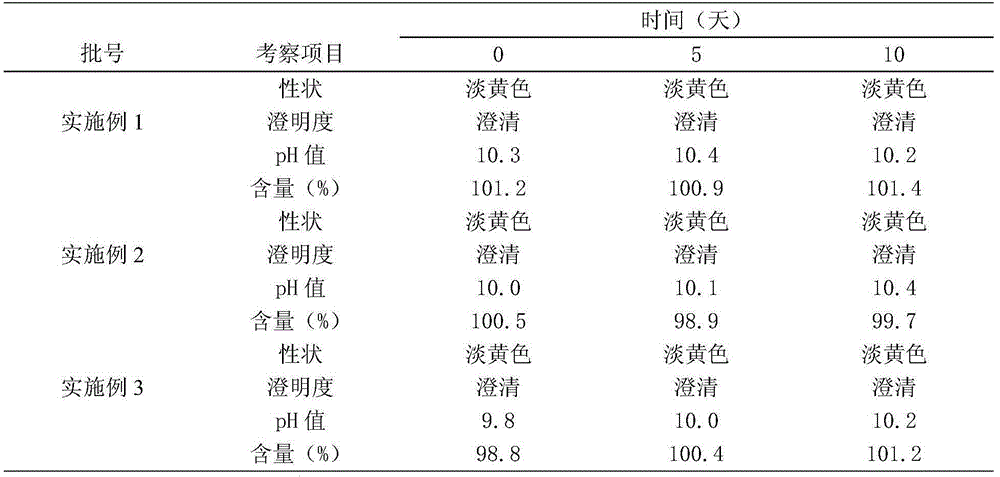
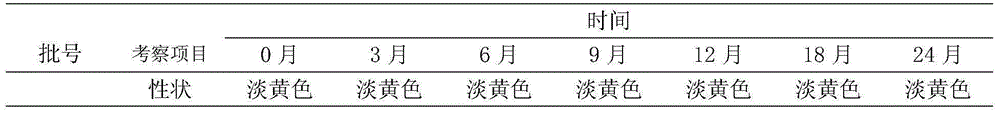
Embodiment 1
[0013] Each 100ml of enrofloxacin injection is prepared by the following steps:
[0014] 1) Weigh 20g of sodium hydroxide and dissolve in 100ml of water for injection to form a 20% sodium hydroxide solution for use;
[0015] 2) Weigh 20g of meglumine and 20ml of polyethylene glycol 400, and dissolve them in 70ml of water for injection at 80°C, respectively, to completely dissolve to obtain a mixed solvent;
[0016] 3) Weigh 20g of enrofloxacin into the mixed solvent, stir to dissolve;
[0017] 4) Add dropwise 20% sodium hydroxide solution until it is clear, stir evenly, make up 100ml of water for injection, and adjust the pH value to 9.5-10.5;
[0018] 5) Coarse filtration, fine filtration, filling with nitrogen, autoclaving at 121°C for 20 minutes, light inspection, packaging.
Embodiment 2
[0020] Each 100ml of enrofloxacin injection is prepared by the following steps:
[0021] 1) Weigh 20g of sodium hydroxide and dissolve in 100ml of water for injection to form a 20% sodium hydroxide solution for use;
[0022] 2) Weigh 15g of meglumine and 15ml of polyethylene glycol 400, and dissolve them in 70ml of water for injection at 80°C, respectively, to completely dissolve to obtain a mixed solvent;
[0023] 3) Weigh 15g of enrofloxacin into the mixed solvent, stir to dissolve;
[0024] 4) Add dropwise 20% sodium hydroxide solution until it is clear, adjust the pH value to 9.5 to 10.5, stir evenly, and make up 100ml of water for injection;
[0025] 5) Coarse filtration, fine filtration, filling with nitrogen, autoclaving at 121°C for 20 minutes, light inspection, packaging.
Embodiment 3
[0027] Each 100ml of enrofloxacin injection is prepared by the following steps:
[0028] 1) Weigh 20g of sodium hydroxide and dissolve in 100ml of water for injection to form a 20% sodium hydroxide solution for use;
[0029] 2) Weigh 10 g of meglumine and 10 ml of polyethylene glycol 400, and dissolve them in 70 ml of water for injection at 80°C, respectively, to completely dissolve to obtain a mixed solvent;
[0030] 3) Weigh 10g of enrofloxacin into the mixed solvent, stir to dissolve;
[0031] 4) Add dropwise 20% sodium hydroxide solution until it is clear, adjust the pH value to 9.5 to 10.5, stir evenly, and make up 100ml of water for injection;
[0032] 5) Coarse filtration, fine filtration, filling with nitrogen, autoclaving at 121°C for 20 minutes, light inspection, packaging.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com