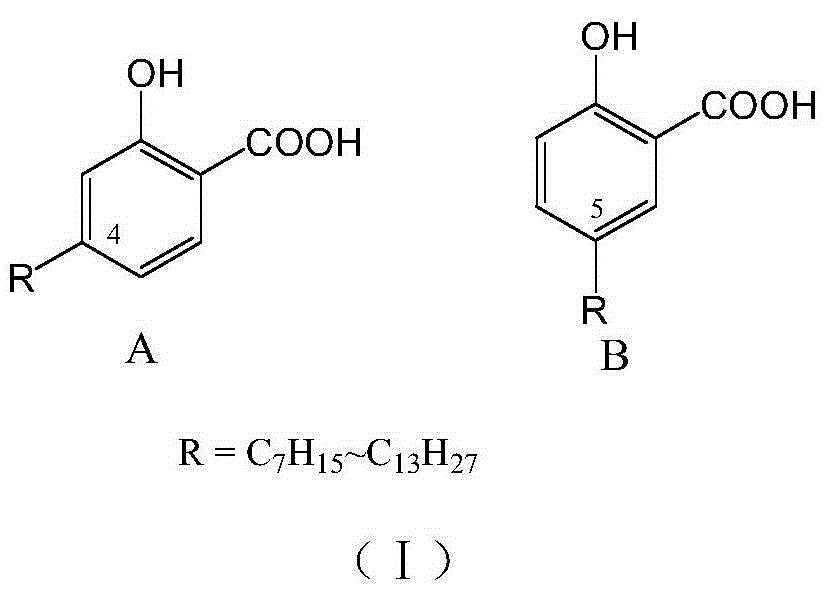
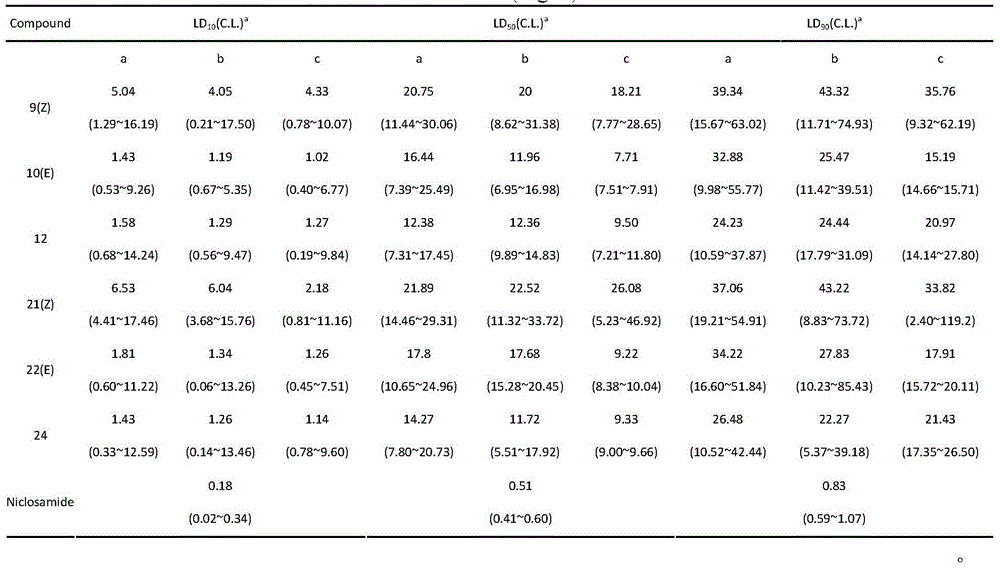
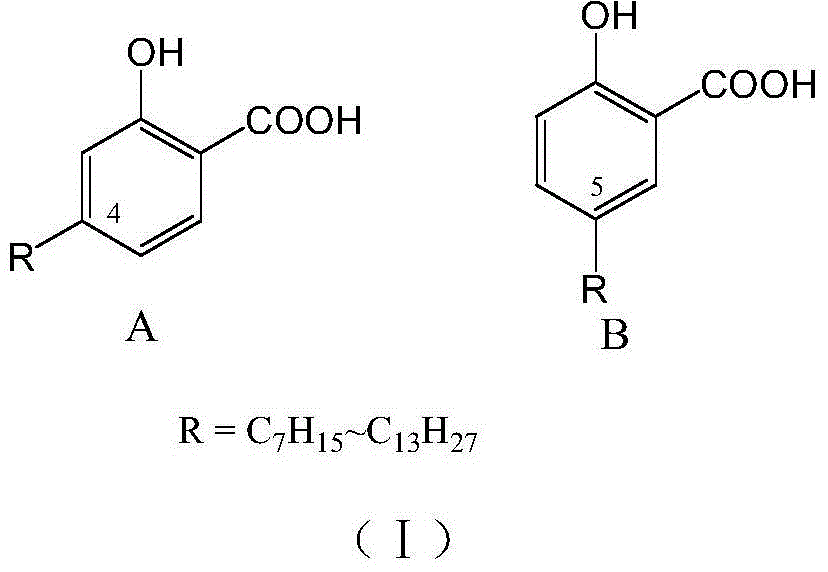
Long-chain alkyl salicylic acid and application thereof in preparing oncomelania killing preparation
A technology for long-chain alkyl salicylic acid and alkyl salicylic acid, which is applied in the field of pesticides and can solve the problems of increased drug resistance, high price, toxicity and the like of target organisms
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Preparation of 2-acetoxy-4-(1-alkyl) methyl benzoate
[0026] 2-Acetoxy-4-(1-enyl) methyl benzoate cis-trans isomer crude product (according to patent CN102010328A synthesis method) is dissolved in acetic acid, and 10% Pd / C of 10% molar weight is added in the solution . Mixed solution room temperature, 60psi with H 2 Rocking reaction 9 ~ 11h. After filtration, the filtrate was poured into water and extracted three times with ethyl acetate. The organic layer was washed with water and saturated brine, respectively, and then washed with Na 2 SO 4 Dry and concentrate under reduced pressure to obtain the crude product. The crude product is subjected to column chromatography (stationary phase is: SilicaGelH mixed uniformly and AgNO with a weight ratio of 1% to 2%. 3 ; The mobile phase was: petroleum ether: ethyl acetate = 50:1) to obtain a pale yellow oil.
Embodiment 2
[0027] Example 2. Preparation of 2-hydroxyl-4-(1-heptyl)benzoic acid
[0028] Methyl 2-acetoxy-4-(1-heptyl)benzoate (88.7 mg, 0.30 mmol) was dissolved in 2.1 mL of ethanol, and 1 mol / L NaOH (0.91 mL, 0.91 mmol) was added dropwise with stirring. The water bath was refluxed for 5h, then the solvent was evaporated, adjusted to PH=3 with 1mol / L HCl, then 5mL of water was added, CH 2 Cl 2 Extract 5mL×3 times, combine the organic layers, anhydrous Na 2 SO 4 Dehydrated and concentrated to give a light yellow powder (67.4mg, 94%). 1 H-NMR (CDCl 3 ,400MHz)δ0.87(t,J=6.5Hz,3H),1.26(m,8H),1.60(m,2H),2.59(t,J=7.4,2H),6.75(d,J=8.3Hz ,1H),6.81(brs,1H),7.80(d,J=8.3Hz,1H),10.41(brs,1H); HRESIMSfound:235.1298.Calcd:235.1334forC 14 h 19 o 3 ([M-H] - ).
Embodiment 3
[0029] Example 3. Preparation of 2-hydroxyl-4-(1-decyl)benzoic acid
[0030] Methyl 2-acetoxy-4-(1-decyl)benzoate (106.8 mg, 0.32 mmol) was dissolved in 2.2 mL of ethanol, and 1 mol / L NaOH (0.96 mL, 0.96 mmol) was added dropwise with stirring. The water bath was refluxed for 6h, then the solvent was evaporated, adjusted to PH=3 with 1mol / L HCl, then 6mL of water was added, CH 2 Cl 2 Extract 6mL×3 times, combine organic layers, anhydrous Na 2 SO 4 Dehydrated and concentrated to give a white powder (80.9 mg, 91%). 1 H-NMR (CDCl 3 ,400MHz)δ0.87(t,J=6.5Hz,3H),1.26(m,14H),1.59(m,2H),2.59(t,J=7.4,2H),6.75(dd,J=8.1Hz ,1.2Hz,1H),6.81(brs,1H),7.80(d,J=8.1Hz,1H),10.41(brs,1H); HRESIMSfound:279.0930.Calcd:279.1955forC 17 h 27 o 3 ([M+H] + ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com