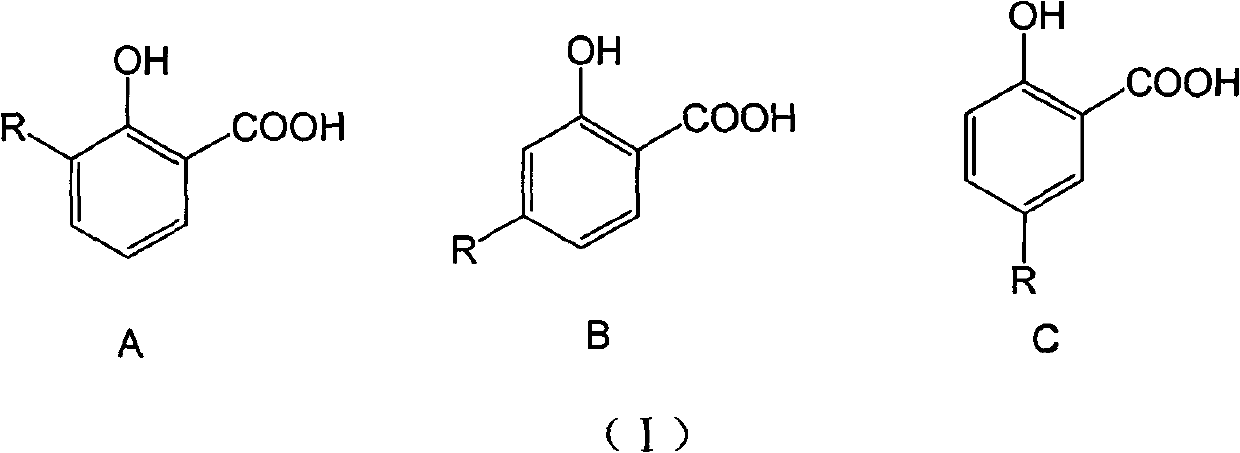
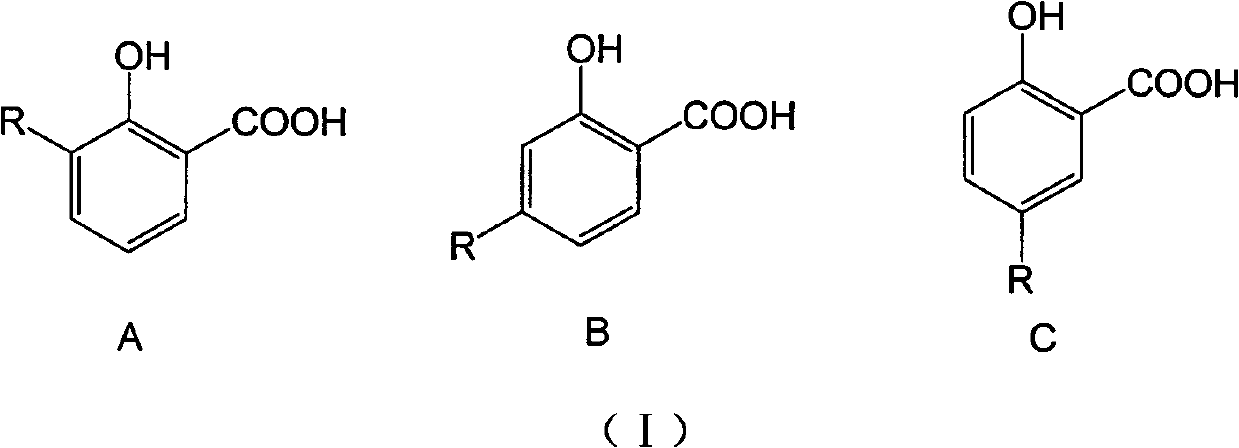
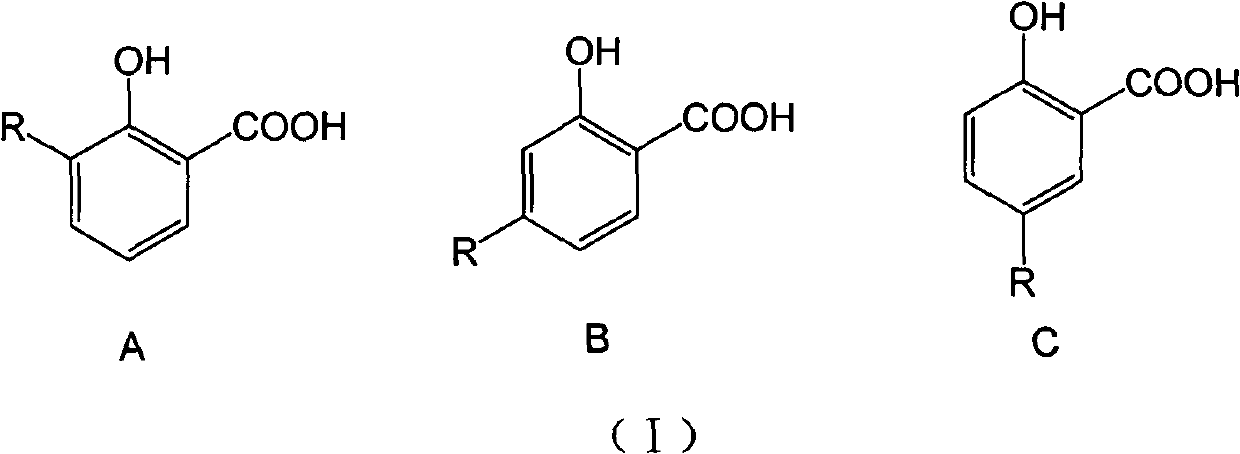
Long-chain alkyl salicylic compound as well as preparation method application thereof
A chain alkyl salicylic acid and alkyl salicylic acid technology, which is applied in the compound synthesis and pesticide fields, can solve the problems of target biological drug resistance increase, environmental pollution, use restriction, etc., and achieve a simple synthesis method, easy promotion, and low cost of use Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] Embodiment 1 synthetic hydrocarbyl salicylic acid compound
[0029] (1). Synthesis of methyl 2-hydroxy-4-methylbenzoate
[0030] 3.5g of 2-hydroxy-4-methylbenzoic acid (21.1mmol) was dissolved in 60mL of methanol, placed in an ice bath, and 12mL of concentrated sulfuric acid was slowly added dropwise. Then the water bath was heated to reflux overnight. Pour into ice water after cooling, extract 100mL x 2 times with dichloromethane, combine the organic layers, and saturated NaHCO 3 Wash the organic layer with anhydrous MgSO 4 After dehydration and concentration, 3.01 g (86%) of light yellow oil was obtained.
[0031]
[0032] (2). Synthesis of methyl 2-acetoxy-4-methylbenzoate
[0033] Add 3g of methyl 2-hydroxy-4-methylbenzoate (18.1mmol) into 8mL of acetic anhydride (8mmol), then add 8 drops of concentrated sulfuric acid dropwise, shake fully, keep in a water bath at 70°C for 10min, and shake occasionally. After cooling down slightly, pour into ice water, extra...
Embodiment 2
[0047] Example 2 Determination of the Activity of Hydrocarbyl Salicylate Compounds with Different Substitution Positions and Carbon Chain Lengths to Kill Oncomelania
[0048] Take 3-, 4-, 5-substituted hydrocarbyl salicylic acid respectively, and the compound whose carbon chain length is 9-13 is an example, and the selected three kinds of different hydrocarbyl salicylic acids are respectively 3-(1-tridecane En)salicylic acid (1), 4-(1-undecene)salicylic acid (2) and 5-(1-nonacene)salicylic acid (3) were synthesized by the above-mentioned method, and the purity was >95% %. In addition, 3-methylsalicylic acid (4), 4-methylsalicylic acid (5) and 5-methylsalicylic acid (6) were used as active references, and the wettability of niclosamide ethanolamine (Nic) The powder is the positive control drug.
[0049] The above monomers were dissolved with a small amount of absolute ethanol (final concentration <0.5%), and then diluted with dechlorinated water to different concentrations: 5...
Embodiment 3
[0058] Embodiment 3 prepares hydrocarbyl salicylic acid microemulsion
[0059] Take 10g of each hydrocarbyl salicylic acid monomer compound prepared above, put it in a 50ml Erlenmeyer flask, dissolve it with an appropriate amount of absolute ethanol, add 10ml of PEG400 and 20ml of water respectively, put it on a magnetic stirrer, stir evenly, and place it on a water bath at 56°C. Use isopropanol placed in a graduated burette to drop the prepared solution until it becomes clear, record the amount of isopropanol consumed, then dilute it with an appropriate amount of water to form a 10% microemulsion, and dilute it with water to a suitable concentration before use.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com