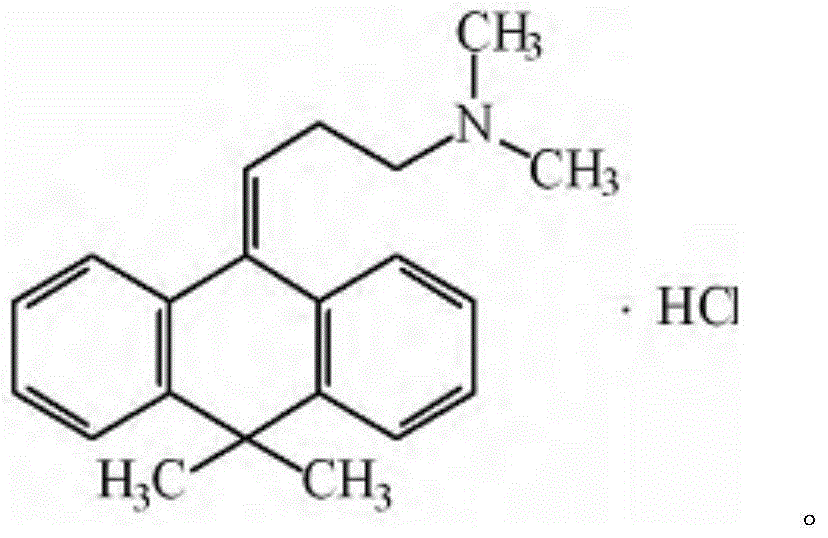
Melitracen hydrochloride compound
A melitracen hydrochloride compound technology, applied in the field of pharmaceuticals, can solve problems such as risks, large benefits, and few adverse reactions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0016] Embodiment 1-compound preparation of the present invention
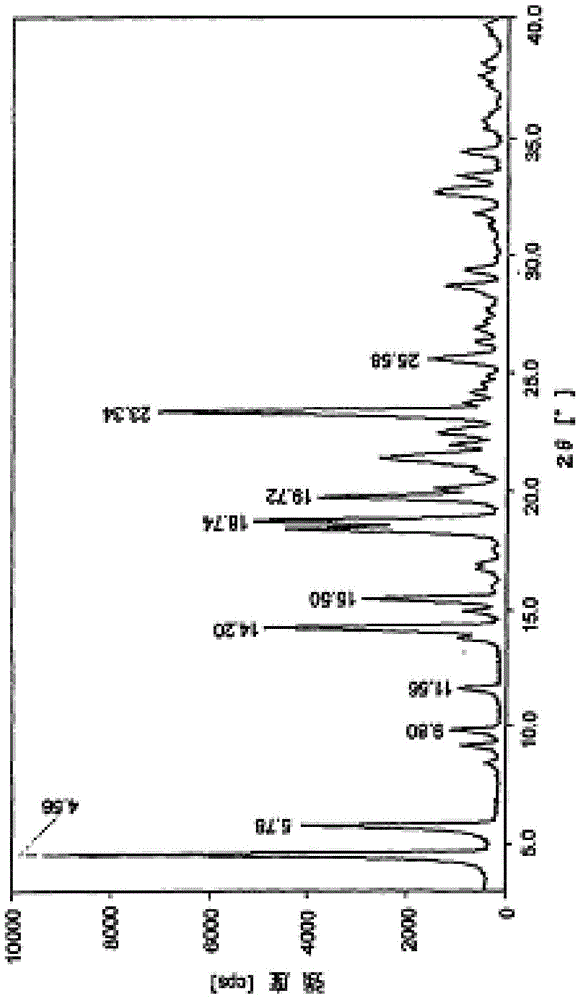
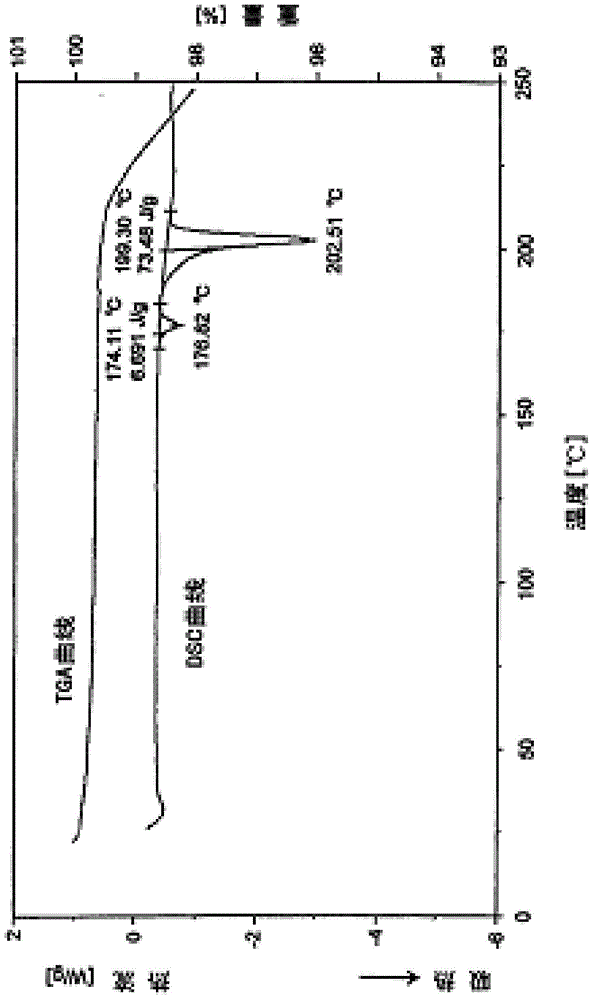
[0017] Melitracen hydrochloride crude product (comparative example 1) 25g, after adding 150ml of acetone, gradually add dropwise 75ml of n-hexane and cyclohexane mixed solvent (the volume ratio of the two is 1:1), fully stir, and precipitate with n-butyl After washing with alcohol for 3 times, the finished melitracen compound was obtained with a yield of 91.7%. Powder X-ray Diffraction: Using Cu-K radiation, the X-ray powder diffraction pattern of this compound is shown in the attached figure 1 . Differential scanning calorimetry analysis: The differential thermal analysis spectrum of this compound is shown in the attached figure 2 .
Embodiment 2
[0018] The formulation of embodiment 2-quality research and quality standard of compound of the present invention
[0019] According to the synthesis process and product characteristics of this product, we have conducted a comprehensive study on the properties, identification, inspection, and content determination of this product with reference to the quality standards for clinical research of this product.
[0020] Properties We have studied the appearance, hygroscopicity and solubility of this product. As a result, the three batches of pilot test samples are white crystalline powder, and this product has no hygroscopicity; according to the results of the solubility investigation of this product, this product is easily Soluble, soluble in methanol or ethanol, slightly soluble in water.
[0021] Inspection In the inspection items, we inspected its acidity, loss on drying, residue on ignition, heavy metals, and the results all met the requirements. According to the synthesis p...
Embodiment 3
[0025] Experimental data of embodiment 3-stability study
[0026] .1. Sample source:
[0027] Influencing factor test sample: batch number: 10021101; batch size: 58g; prepared by Sichuan Haisco Pharmaceutical Co., Ltd.; test time: February 2010. Samples for stability testing: batch numbers: 10022701, 10022702, 10022703. The batches are 99.3g / batch, 99.8g / batch, 103.1g / batch; prepared by Sichuan Haisco Pharmaceutical Co., Ltd.; test time: February 2010.
[0028] .2 Inspection items
[0029] According to the second appendix "Guiding Principles of Drug Stability Test" of the 2010 edition of "Chinese Pharmacopoeia", according to the inspection method stipulated in the quality standard (draft) of this product, the appearance, acidity, loss on drying, related substances, content, etc. of this product were mainly investigated. The project is inspected.
[0030] 3. Illumination test
[0031] Take an appropriate amount of this product (10021101), place it under the condition of 45...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com