A kind of preparation method of melitracen hydrochloride
A technology of melitracen hydrochloride and dimethyl anthracene, which is applied in the field of drug preparation, can solve problems such as inability to meet large-scale production, low yield of melitracen, and achieves low cost, high purity and high yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] A preparation method of melitrazin hydrochloride includes the following steps:
[0045] (1) Preparation of melitrazin intermediate
[0046] 10,10-Dimethylanthracene-9-one and 3-dimethylamino-1-chloropropane undergo a Grignard reaction in the presence of an initiator to obtain a melitrazin intermediate. The specific process is as follows:
[0047] Add 340g of magnesium bar and 17.5L of anhydrous ether into a 20L glass reactor, stir and heat to 30~35℃, add 1.75kg of 3-dimethylamino-1-chloropropane, add 1g of iodine and 2mL 1 ,2-Dibromoethane was used as the initiator, stirred and refluxed for 9h, the magnesium bars disappeared completely, the reaction system was cooled to 10-20℃, 1.5kg 10,10-dimethylanthracene-9-one was slowly added, and the temperature was raised to The reaction was refluxed at 30~35℃ for 1 hour; TLC monitored the reaction to be complete, the reaction system was cooled to 10~20℃, and 5.5L of water was added, the ether layer was separated, and anhydrous sodium ...
Embodiment 2
[0058] A preparation method of melitrazin hydrochloride includes the following steps:
[0059] (1) Preparation of melitrazin intermediate
[0060] This step is the same as step (1) in Example 1;
[0061] (2) Preparation of crude melitrazin
[0062] This step is the same as step (2) in embodiment 1;
[0063] (3) Purification of crude meritrazine
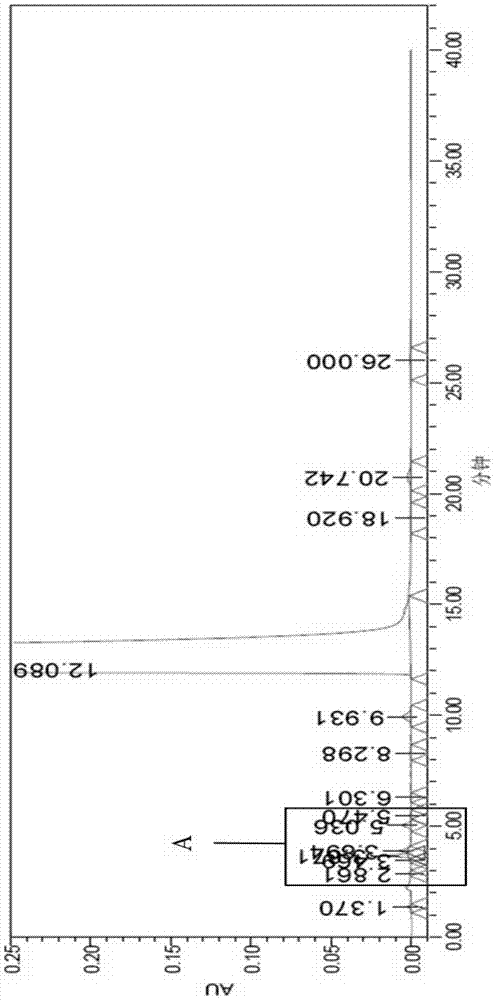
[0064] Take 10g of crude melitrexine (purity 99.41%, formula I: 0.20%, formula II: 0.13%), add 4 times the amount (W / V) of ethanol, stir at 20~25°C for 4h (beating), filter and dry , The product obtained is 9.79g, the yield is 97.9%, the purity is 99.69%, the formula I is 0.047%, and the formula II is 0.005%; the liquid phase spectrum of the crude melitrazin sample after ethanol beating is shown in the attachment Image 6 ;
[0065] Add 9.0g of ethanol-beaten product into a 250mL round-bottomed flask, then add 230mL of ethanol to reflux to dissolve, then cool to 10°C and agitate to crystallize overnight, filter with suction, and dry under reduce...
Embodiment 3
[0067] A preparation method of melitrazin hydrochloride includes the following steps:
[0068] (1) Preparation of melitrazin intermediate
[0069] This step is the same as step (1) in Example 1;
[0070] (2) Preparation of crude melitrazin
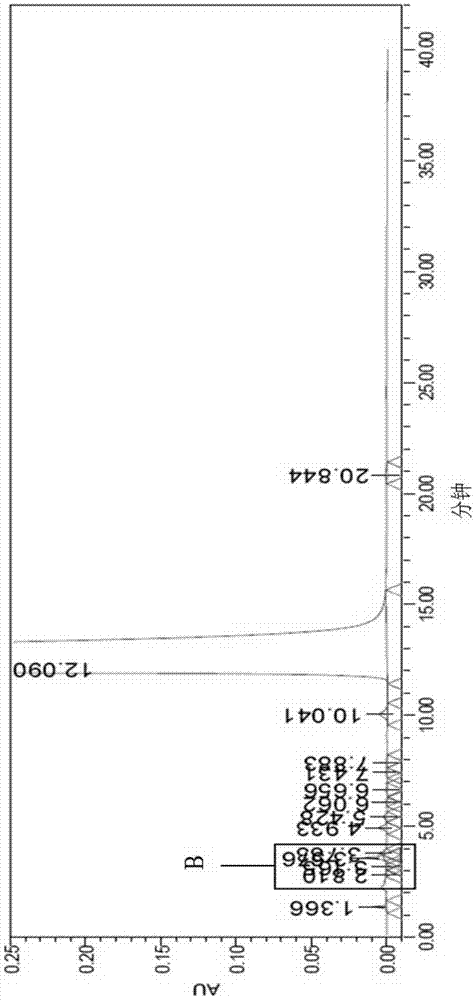
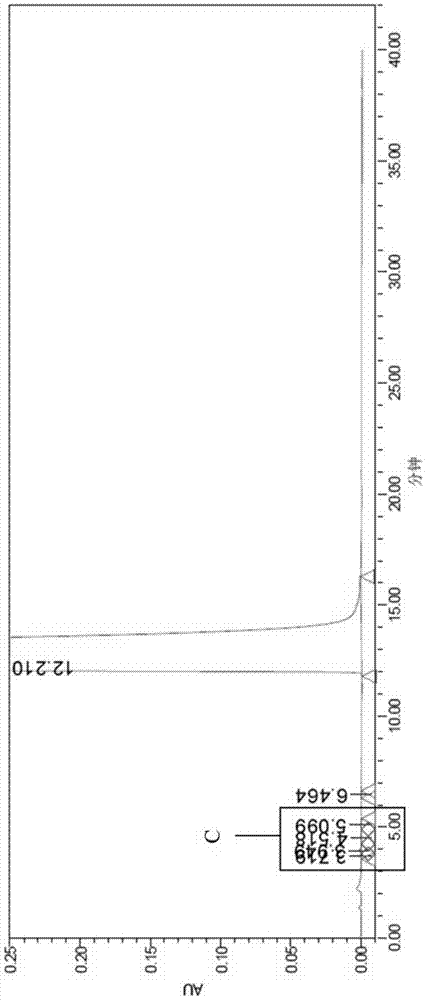
[0071] Put 100g of melitrazin intermediate, 500mL of chloroform and 120mL of concentrated hydrochloric acid into a 1L three-necked flask, stir to dissolve to obtain a light yellow solution, raise the temperature to 60℃, stir and react for 2 hours, TLC monitors the reaction to complete, separate the water layer, organic The phase was concentrated and dried under reduced pressure to obtain 104g of the white solid, which is the crude melitrazin product, with a yield of 98.3% and a purity of 99.38%, containing formula I: 0.22%, formula II: 0.15%; Attached Figure 8 ;
[0072] TLC monitoring method: take the organic phase of the reaction liquid to a dot plate, and the developing solvent is dichloromethane: methanol: acetic acid = 150: 10: 2 (volume rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com