A kind of synthetic method of stable deuterium-labeled melitracen hydrochloride
A technique for the synthesis of melitracen hydrochloride and its synthesis method, which is applied in the field of synthesis of melitracen hydrochloride, achieving the effects of reasonable process design, short route and strong operability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
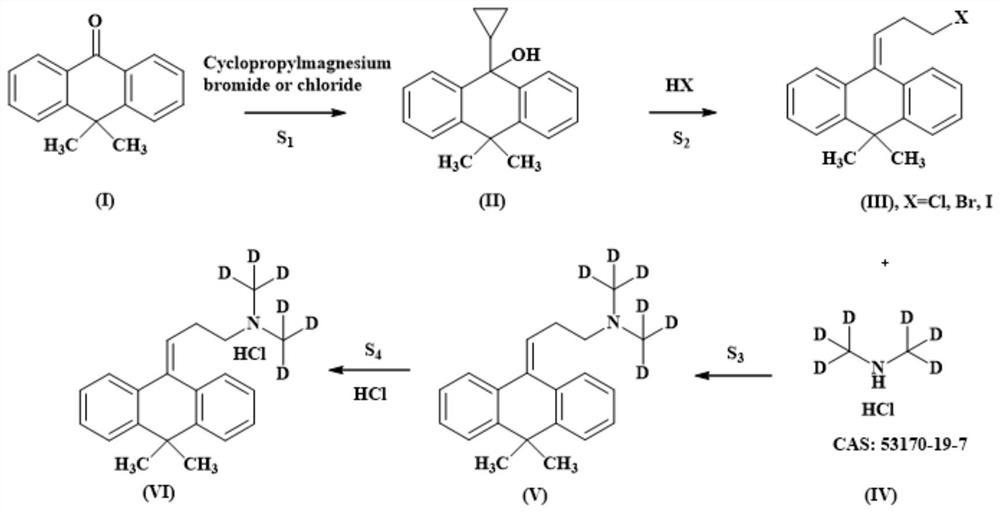
[0030] Such as figure 1 Shown, a kind of synthetic method of the melitracen hydrochloride of stable deuterium label comprises the following steps:
[0031] (1) Take 20.0 g of commercially available 10,10-dimethylanthrone I, add it to a 500 mL round bottom flask, dissolve it with 100 mL of dry ether, add commercially available cyclopropylmagnesium bromide (1M in THF) , After the dropwise addition, react at 35°C for 12 hours; TLC board monitors the complete reaction of the raw materials, process and purify, and obtain 21.2g of intermediate II, MS: 265.2[M+1] + , yield 89.25%;
[0032]
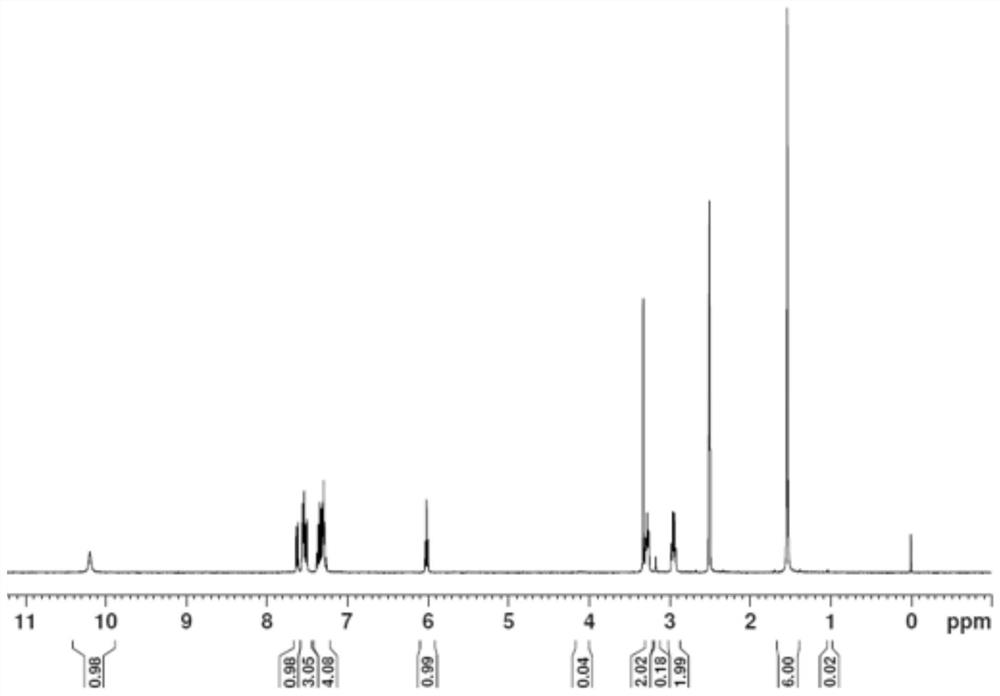
[0033] (2) Dissolve 20.0 g of intermediate II in 80 mL of formic acid, slowly add 48% hydrobromic acid under ice-bath conditions, and then react at 60°C for 8 hours. TLC board monitors that the reaction of the raw materials is complete, process them out, and purify by column chromatography , to obtain 22.0g of intermediate III, the yield is 88.86% (X=Br); 1H NMR (400MHz, CDCl3) of intermedia...
Embodiment 2
[0040] Such as figure 1 Shown, a kind of synthetic method of the melitracen hydrochloride of stable deuterium label comprises the following steps:
[0041] (1) Take 20.0g of commercially available 10,10-dimethylanthrone I, put it into a 500mL round bottom flask, dissolve it with 100mL of dry tetrahydrofuran, add dropwise 130mL of commercially available cyclopropylmagnesium chloride (1M in THF) , after the dropwise addition was completed, the oil bath was reacted at 60 degrees for 6 hours; the TLC board monitored the reaction of the raw materials, quenched the reaction, and purified by column chromatography to obtain 18.0 g of intermediate II, which was a light brown oil with a yield of 75.78%; The structure of II is as follows, MS: 265.2[M+1] + .
[0042]
[0043] (2) Dissolve 10.0 g of intermediate II in 40 mL of acetic acid, slowly add 15 mL of commercially available concentrated hydrochloric acid with a concentration of 36% under ice-bath conditions, and then react at ...
Embodiment 3
[0050] Such as figure 1 Shown, a kind of synthetic method of the melitracen hydrochloride of stable deuterium label comprises the following steps:
[0051] (1) Take 20.0g of commercially available 10,10-dimethylanthrone I, put it into a 500mL round bottom flask, dissolve it with 100mL of dry tetrahydrofuran, add dropwise commercially available cyclopropylmagnesium bromide (1M in THF ) 120mL, after the dropwise addition, put the oil bath at 50 degrees to react for 8 hours; the TLC board monitors the reaction of the raw materials completely, quenches the reaction, and purifies by column chromatography to obtain 19.2g of intermediate II, which is a light brown oil, with a yield of 80.83% ; The structure of intermediate II is as in Example 1, MS: 265.2[M+1] + .
[0052]
[0053] (2) Dissolve 15.0g of intermediate II in 75mL of ethanol, slowly add 15mL of commercially available hydriodic acid with a concentration of 55% under ice bath conditions, and then react at 60°C for 24 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com