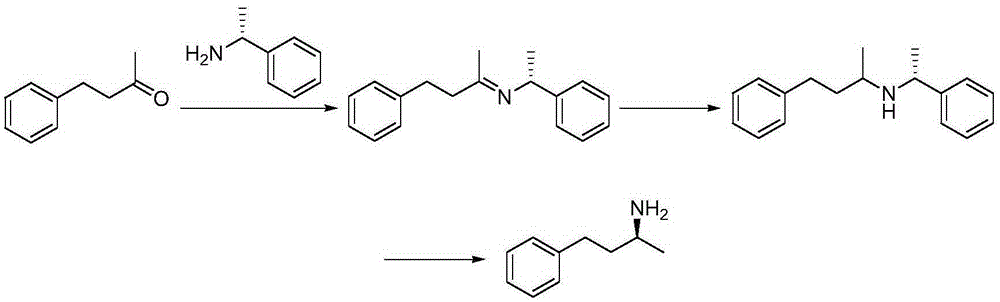
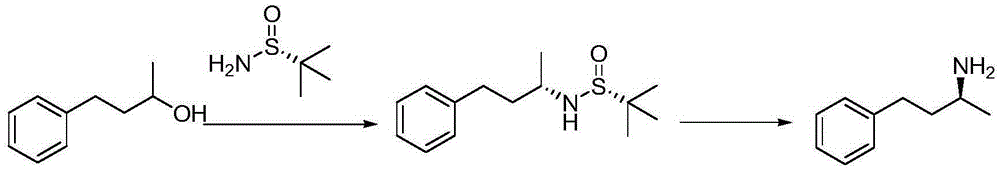
Synthesis method of (R)-(-)-1-methyl-3-amphetamine
A synthetic method and technology of amphetamine, which is applied in the field of synthesis of the key intermediate - 1-methyl-3-amphetamine, can solve the problems of high cost, unsuitability for industrial production, harsh synthesis conditions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
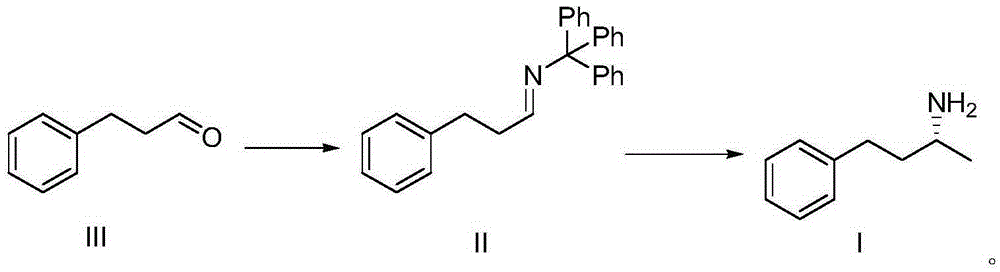
[0020] Synthesis of compound II:
[0021]
[0022] Add phenylpropionaldehyde (55g, 0.41mol) to tetrahydrofuran (400ml), then add tritylamine (106.2g, 0.41mol) and tetrabutyl titanate (278.8g, 0.82mol), and stir at room temperature for 1 hour , and then refluxed for 4 hours, TLC spot plate detected that the reaction of the raw materials was complete, cooled to room temperature, added saturated sodium bicarbonate, stirred, stood still, separated into layers, separated the organic phase, concentrated under reduced pressure, and added saturated saline and ethyl acetate to the residue Esters, extraction, standing to separate layers, separating the organic phase, drying with anhydrous sodium sulfate, filtering to remove the desiccant, concentrating under reduced pressure to obtain the crude product of compound II, using ethyl acetate:petroleum ether=3:7 (volume ratio ) recrystallization to obtain off-white solid compound II99g, yield 71%.
[0023] 1HNMR (400MHz, CDCl 3 ): δppm7...
Embodiment 2
[0029] Synthesis of compound II:
[0030]
[0031] Add phenylpropionaldehyde (55g, 0.41mol) to toluene (400ml), then add tritylamine (106.2g, 0.41mol) and tetrabutyl titanate (278.8g, 0.82mol), and stir at room temperature for 1 hour , and then refluxed for 4 hours, TLC spot plate detected that the reaction of the raw materials was complete, cooled to room temperature, added saturated sodium bicarbonate, stirred, stood still, separated into layers, separated the organic phase, concentrated under reduced pressure, and added saturated saline and ethyl acetate to the residue Esters, extraction, standing to separate layers, separating the organic phase, drying with anhydrous sodium sulfate, filtering to remove the desiccant, concentrating under reduced pressure to obtain the crude product of compound II, using ethyl acetate:petroleum ether=3:7 (volume ratio ) recrystallized to obtain 103.1 g of off-white solid compound II, with a yield of 74%.
[0032] 1HNMR (400MHz, CDCl 3 )...
Embodiment 3
[0038] Synthesis of compound II:
[0039]
[0040] Add phenylpropionaldehyde (55g, 0.41mol) to 1,4-dioxane (450ml), then add tritylamine (106.2g, 0.41mol) and tetraethyl titanate (187g, 0.82mol) , stirred at room temperature for 1 hour, and then refluxed for 4 hours. The reaction of the raw materials was detected by TLC spotting. Saturated saline and ethyl acetate, extraction, standing to separate layers, separating the organic phase, drying with anhydrous sodium sulfate, filtering to remove the desiccant, concentrating under reduced pressure to obtain the crude product of compound II, using ethyl acetate:petroleum ether= 3:7 (volume ratio) recrystallization to obtain off-white solid compound II96g, yield 68.9%.
[0041] 1HNMR (400MHz, CDCl 3 ): δppm7.49~7.31(m,5H), 7.07~6.92(m,15H), 6.84(m,1H), 2.63(t,2H), 1.8(t,2H); m / z=376(M +H)+.
[0042] Synthesis of (R)-(-)-1-methyl-3-amphetamine:
[0043]
[0044] Compound II (37.5g, 0.1mol) was added to diethyl ether (500ml), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com