Porcine pseudorabies virus vaccine composition and preparing method and application thereof
A technology of porcine pseudorabies and vaccine composition, applied in the field of porcine pseudorabies virus vaccine composition and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] The preparation of embodiment 1 porcine pseudorabies virus gB, gD protein
[0072] 1. Amplification of porcine pseudorabies virus gB and gD genes
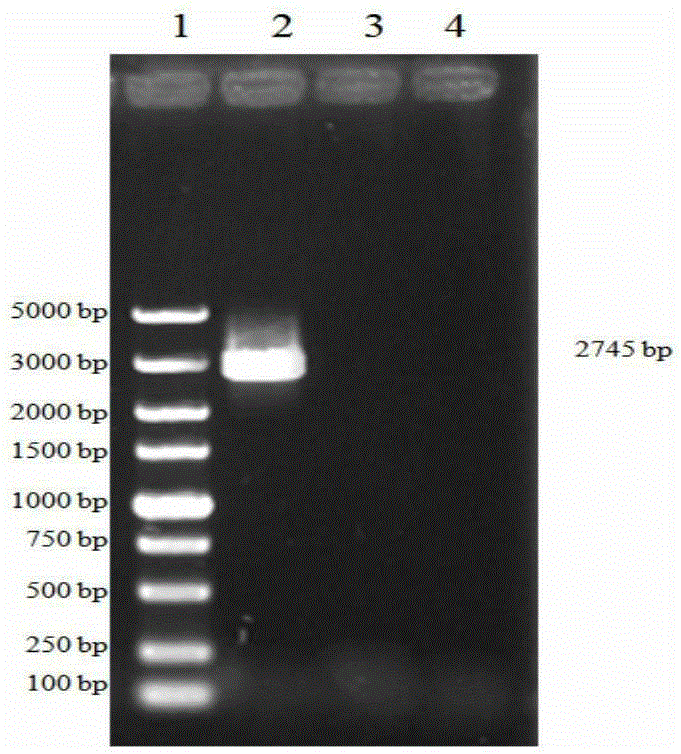
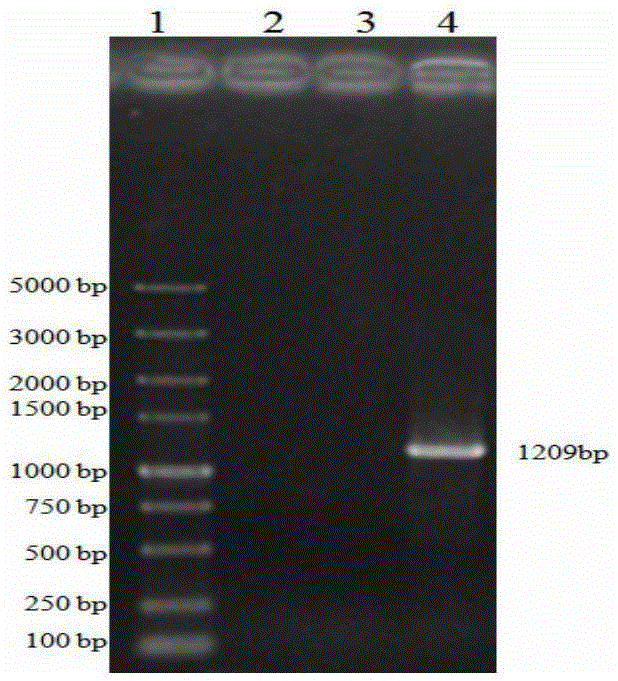
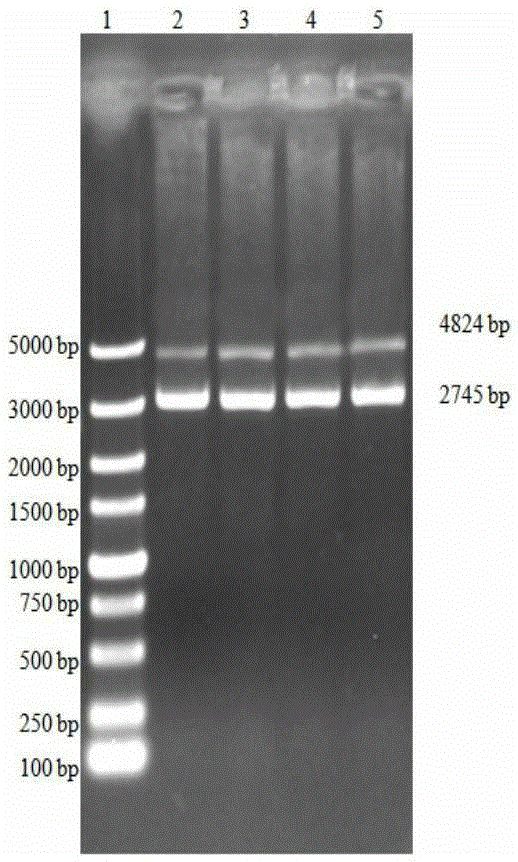
[0073]Inoculate PRVHN1201 virus or cultures of different generations on well-growing PK15 cells. The cultures of different generations are cultures within 5-35 generations. After harvesting the virus, extract it with the MiniBESTViralRNA / DNAExtractionKitVer.3.0 kit from TAKARA Company PRV genomic DNA. Take 1 μl of genomic DNA as a template, and use gB and gD specific primers respectively:
[0074] gBSF: 5'CTAGGGGGCGTCGGGGTCCTCGT3' and
[0075] gBSR: 5′ATGCCCGCTGGTGGCGGTCTTTGG3′
[0076] gDSF: 5'ATGCTGCTCGCAGCGCTATTGGC3' and
[0077] gDSR: 5′CTACGGACCGGGCTGCGCTTTTAG3′
[0078] Perform PCR amplification using TAKARA's high-fidelity enzymes HSDNAPolymerasewithGCBuffer, gB amplification conditions are: 94°C for 3min; 98°C for 10s, 68°C for 3min, 30cycles; 68°C for 5min, the PCR product is named as gB. The gD amplificatio...
Embodiment 2
[0087] Implementation 2 Preparation of porcine pseudorabies virus vaccine composition
[0088] Take the gB and gD proteins prepared in Example 1, slowly add them to the adjuvant, and continuously stir with an emulsifier at 800 rpm for 12 minutes during the addition process, mix well, and store at 4°C, which is the vaccine composition of porcine pseudorabies virus . See Table 1 for specific ratios.
[0089] Table 1 Porcine pseudorabies virus vaccine composition ratio
[0090]
[0091]
Embodiment 3
[0092] The immunogenicity test of embodiment 3 porcine pseudorabies virus vaccine composition
[0093] 36 PRV antibody-negative piglets at the age of 21 days were randomly divided into 9 groups, 4 heads / group, that is, 1-7 groups were respectively vaccine 1, vaccine 2, vaccine 3, vaccine 4, vaccine 5, and vaccine prepared in Example 1 of the present invention. 6. Vaccine 7 immunization group, the 8th group and 9th group were injected with the same amount of PBS, single immunization. 28 days after immunization, the virus was challenged, and the dose of the virus challenge was 2×10 porcine pseudorabies virus HN1201 strain 8.0 TCID 50 / head, observe the clinical condition and measure the body temperature at a fixed time every day for 7 days after the challenge.
[0094] Results Under the challenge dose, the 4 piglets in the 1st to 7th immunization groups were all protected, showed transient clinical signs and gradually returned to normal after 5 days, and finally survived. Di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com