A matrine derivative with antitumor properties
A technology of derivatives, matrine, applied in the field of derivatives, can solve the problems of low therapeutic efficiency, poor selectivity, and cannot be widely used in clinical practice, and achieves the effect of improving anti-tumor activity and expanding types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
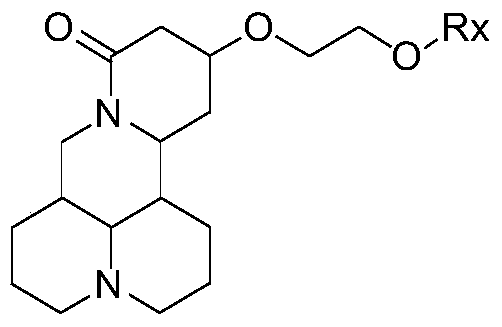
[0047] Method for preparing matrine derivative represented by general formula I:
[0048] Preparation of 13-(2-hydroxy)ethoxymatrine (Compound 1)
[0049]
[0050] Add 30mL dry ethylene glycol to a dry 100mL three-necked flask, N 2 Under the protection of the atmosphere, 0.10 g of sodium wire was put in, the temperature was raised to 50°C, and the reaction was stirred. When the sodium wire disappears completely, stop heating and cool to room temperature. Dissolve 2.00g sophocarpine in 10mL ethylene glycol, slowly drop it into the above reaction flask, gradually increase the temperature to 60℃, follow the reaction progress by TLC, after 23h, add 10mL water to the reaction solution to stop the reaction, and extract three times with 15mL chloroform , Combined extracts, anhydrous Na 2 SO 4 Dry overnight, filter, and concentrate under reduced pressure to obtain a crude yellow oil. Elution was carried out with a mixture of ethyl acetate and ethanol in a volume ratio of 80:1, and 1.72 g...
Embodiment 2
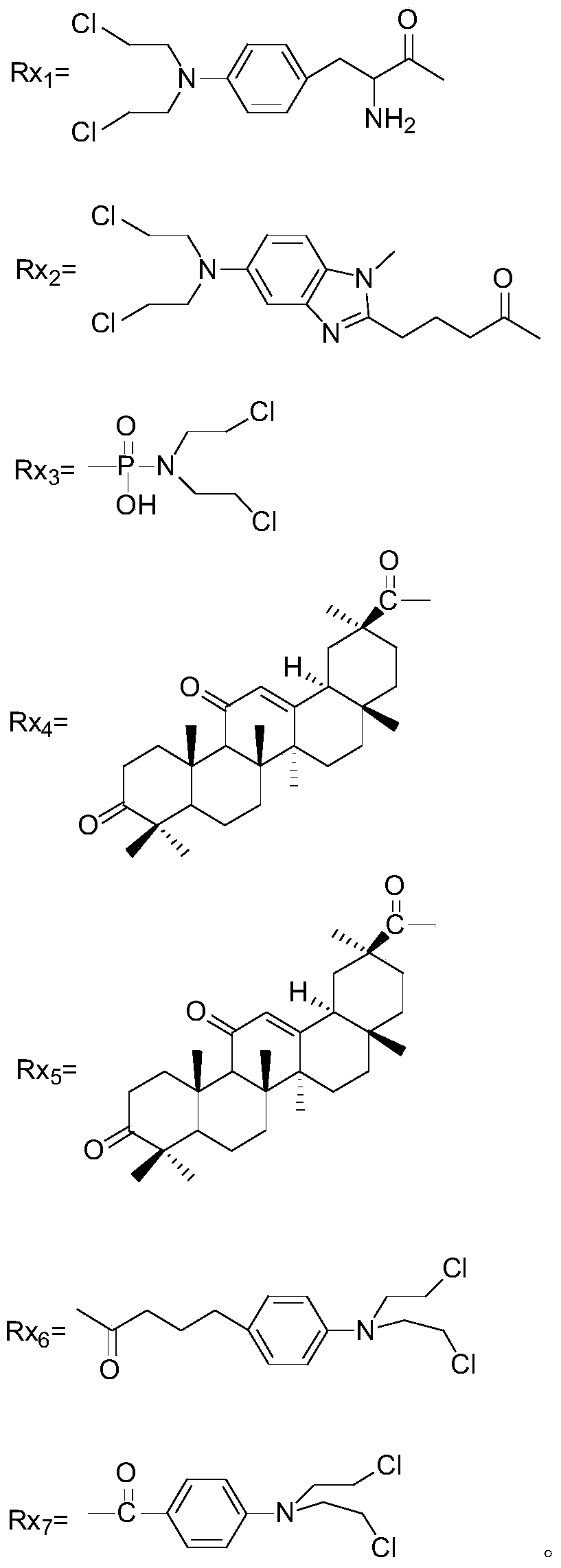
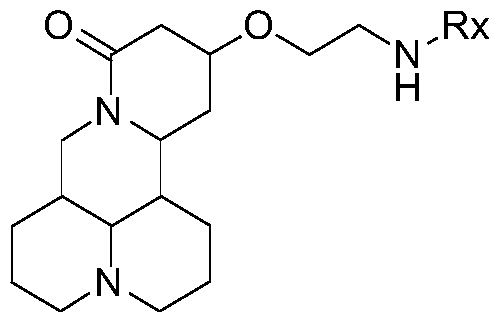
[0086] Preparation method of matrine derivatives of general formula II
[0087] Preparation of 13-(2-amino)ethoxymatrine (Compound 2)
[0088]
[0089] Add 30mL dry ethanolamine to a dry 100mL three-necked flask, N 2 Under the protection of the atmosphere, 0.10 g of sodium wire was put in, the temperature was raised to 50°C, and the reaction was stirred. When the sodium wire disappears completely, stop heating and cool to room temperature. Dissolve 2.00 g sophocarpine in 10 mL ethanolamine, slowly drop it into the above reaction flask, gradually increase the temperature to 60°C, and follow the reaction process by TLC. After 23 hours, the raw materials have reacted completely and undergo post-processing. Add 10 mL of water to the reaction solution to stop the reaction, extract three times with 15 mL of chloroform, combine the extracts, anhydrous Na 2 SO 4 Dry overnight, filter, and concentrate under reduced pressure to obtain a crude yellow oil. Elution was carried out with a mixt...
Embodiment 3
[0114] Preparation method of matrine derivatives of general formula Ⅲ
[0115] Preparation of Matrine
[0116]
[0117] Add 4.96g (0.02mol) matrine, 50mL absolute ethanol, 7.5g (0.1875mol) NaOH solid to a 100mL three-necked flask, and react at 105°C. TLC detects the progress of the reaction. After 6h, the raw materials have reacted completely. , Post-processing. After the reaction liquid is cooled, it is placed in an ice bath, the reaction liquid is neutralized with dilute sulfuric acid, the sodium sulfate is removed by suction filtration, the liquid is collected, and concentrated to dryness to obtain 4.30 g of white solid, which is matrine. The yield is 80.83%, mp197~198℃ (literature value [6] : 197.7~198.5℃).
[0118] Preparation of Ethyl Matrine (Compound 3)
[0119]
[0120] Add 1.33 g (0.005 mol) of matrine and 40 mL of absolute ethanol to a 100 mL single-necked flask, add 3.0 mL of thionyl chloride dropwise at 0°C, stir for 0.5h, and reflux at 65°C for 4h. The reaction soluti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com