Imidazopyridine-6-formyl-Met-AA-OBzl, synthesis, activity and applications thereof
A technology of imidazo and pyridine, which is applied in the field of antithrombotic and anti-inflammatory drugs, and can solve problems such as worsening prognosis of tumor patients and endangering health
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
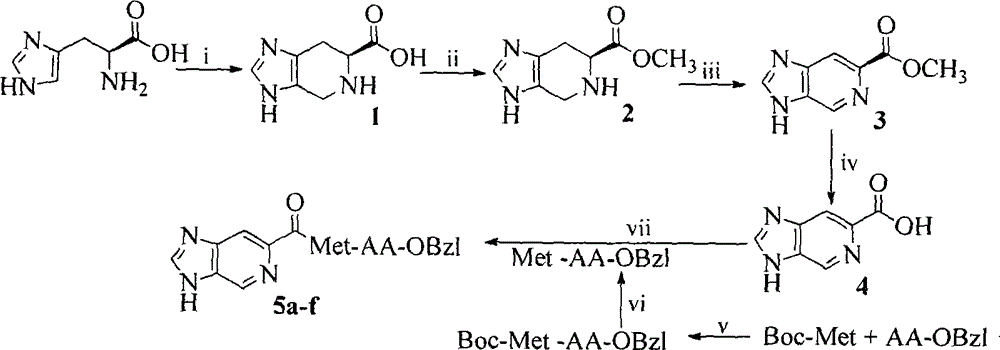
[0019] Preparation of Example 1 Compound 6S-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxylic acid (1)
[0020] Mix 10.00g (64.5mmol) L-histidine, 30mL water in a 100mL eggplant bottle, add 2mL concentrated sulfuric acid drop by drop with a constant pressure funnel under ice bath, with the addition of concentrated sulfuric acid, the raw material gradually dissolves, and wait until it is completely dissolved Finally, 10 mL of 40% formaldehyde solution was added to the reaction liquid, and reacted at 65° C. for 5 hours in a microwave accelerated reaction apparatus. After the reaction was completed, concentrated ammonia water was slowly added dropwise in an ice bath to adjust the pH of the solution to 6-7, a large amount of white precipitates were precipitated, and filtered under reduced pressure to obtain 10.76 g (99.2%) of a colorless solid. ESI-MS(m / z): 168[M+H] + .
Embodiment 2
[0021] Example 2 Preparation of Compound 6S-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxylic acid methyl ester (2)
[0022] Add 15.6mL of methanol to a 100mL eggplant bottle under ice-cooling, slowly add 1.56mL of thionyl chloride dropwise with a constant pressure funnel under ice-cooling, and add 1.00g (6mmol) of 6S-4,5,6,7-tetrahydro -3H-imidazo[4,5-c]pyridine-6-carboxylic acid (2), after 3 days of reaction at room temperature, the reaction was complete as monitored by TLC, the reaction mixture was concentrated under reduced pressure, and the residue was dissolved in methanol and concentrated under reduced pressure. This operation was repeated 3 times to obtain a white foamy solid, which was then drained with diethyl ether and repeated 3 times to obtain a colorless powder, and finally recrystallized from methanol / diethyl ether to obtain 0.58 g (53%) of the title compound as a colorless solid. ESI-MS(m / z)181[M+H] + .
Embodiment 3
[0023] Preparation of Example 3 Compound 3H-imidazo[4,5-c]pyridine-6-carboxylic acid methyl ester (3)
[0024] Add 1.1g (6.1mmol) 6S-4,5,6,7-tetrahydro-3H-imidazo[4,5-c]pyridine-6-carboxylic acid methyl ester (3) in a 100mL eggplant bottle under ice-cooling, add DMF dissolves. 1 mL of triethylamine was added dropwise to the solution to adjust the pH to 8, and 1.2 g (7.6 mmol) of potassium permanganate was added three times. After reacting for 6 hours, TLC monitored the completion of the reaction. The reactant was blown to dryness, and the obtained black solid was dissolved in 1N HCl solution, and 2N NaOH solution was added dropwise in ice bath to adjust the pH to 7, and a large amount of colorless solid was precipitated. The solid was purified on a silica gel column eluting with dichloromethane / methanol to afford 0.68 g (63.2%) of the title compound as a colorless solid. ESI-MS(m / z)177[M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com