Polycation inclusion complex, and preparation method and purpose thereof
A technology of polycation and inclusion compound, which is applied in the directions of non-active components of polymer compounds, drug combinations, pharmaceutical formulations, etc., can solve the problem that it is difficult to meet the requirements of stable cell dissociation and release, lack of selectivity, application scope and use effect limitations. And other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1: the synthesis of β-cyclodextrin initiator
[0058] Weigh 4.5 mmol of β-cyclodextrin, dissolve it in 30 ml of anhydrous N,N-dimethylacetamide under stirring, and add 22.5 mmol of triethylamine. Weigh 22.3mmol of 2-bromoisobutyryl bromide and dissolve it in 10ml of anhydrous N,N-dimethylacetamide. The 2-bromoisobutylacyl bromide solution was slowly added dropwise to the β-cyclodextrin solution, stirred in an ice bath, then slowly raised to room temperature, and continued to stir overnight. After the reaction was completed, precipitate with ether and use acetone to obtain 3.3 g of 4-substituted β-cyclodextrin initiator (yield: 42.4%). 1 HNMR (CDCl 3 ): δ=1.88(24H,-OCO-C(CH 3 ) 2 Br), 3.00-6.00 (66H, -OH and -CH-β-CD).
Embodiment 2
[0059] Embodiment 2: the synthesis of adamantane initiator
[0060] Weigh 3.0 g (18.0 mmol) of adamantane methanol and dissolve it in 50 ml of dichloromethane, and then add 3 ml (21.6 mmol) of triethylamine to the solution. Measure 2.7ml (21.8mmol) of 2-bromoisobutyryl bromide, dissolve it in dichloromethane, and add it dropwise to the adamantane methanol solution under ice-cooling. After the dropwise addition, the stirring was continued overnight. After the solution was concentrated by rotary evaporation, the product was purified with a silica gel chromatography column to obtain 4.6 g of adamantane initiator (yield: 81%). 1 HNMR (CDCl 3 ): δ=2.00 (3H, CH), 1.95 (6H, CH 2 ), 1.69 (6H, -C (CH 3 ) 2 Br), 1.58 (6H, CH 2 ).
Embodiment 3
[0061] Example 3: Synthesis of polydiisopropylaminoethyl methacrylate modified β-cyclodextrin
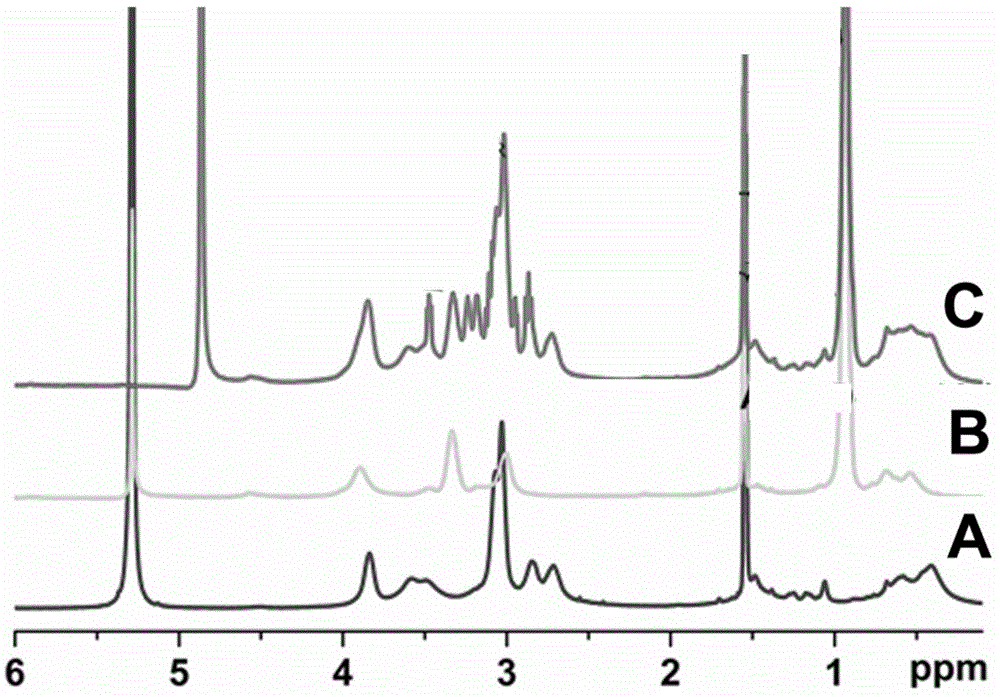
[0062] Take 0.3 g of the β-cyclodextrin initiator synthesized in Example 1 and dissolve in 1.5 ml N,N-dimethylacetamide and stir until dissolved, add 1 ml of diisopropylaminoethyl methacrylate (DPMA) and catalyst Pentamethyldiethylenetriamine (PMDETA) 132μl, add catalyst cuprous bromide 90mg under anaerobic conditions, stir and react at 40°C for 24 hours, peroxide column, obtain polydiisopropylaminoethyl methacrylate modified β-cyclodextrin 1.14 g (yield: 83%). 1 HNMR(DMF): δ=1.88-2.25(44H,-OCO-C(CH 3 ) 2 -CH 2 -CCH 3 Br), 2.74(8H, CH), 3.08(8H, CH 2 ), 3.99 (8H, CH 2 ), 3.00-6.00 (66H, -OH and -CH-β-CD). Its NMR data are as figure 1 shown in B.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com