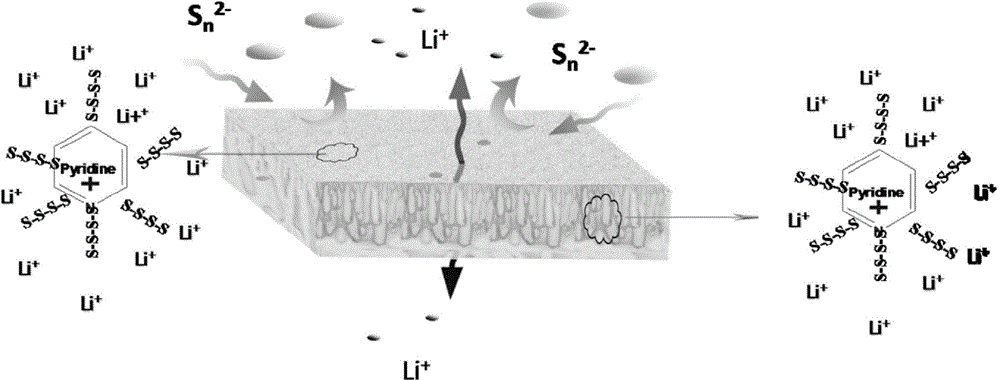
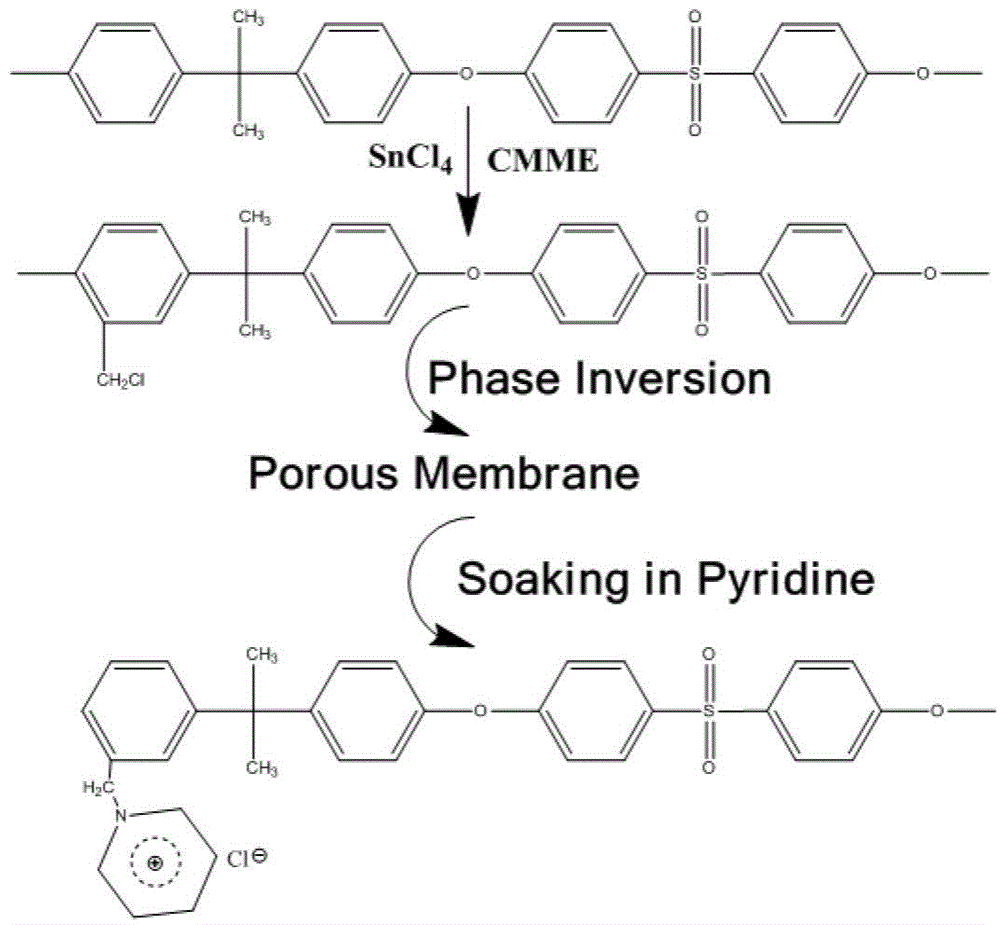
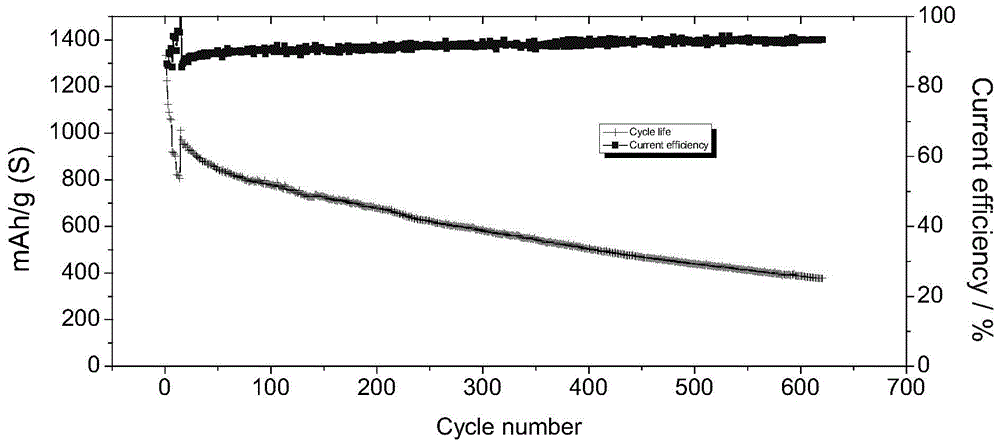
Application of alkaline porous membrane in lithium-sulfur rechargeable battery
A lithium-sulfur secondary battery, porous membrane technology, applied in battery pack parts, circuits, electrical components, etc., can solve the problems affecting the cost and service life of lithium-sulfur batteries, high cost, difficult practical application, etc. Selective permeability, low cost, and controllable effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] 2g of chloromethyl polysulfone (the degree of chloromethylation is 135mmol / g) is dissolved in 8g of DMAC, stirred for 5 hours, and the polymer solution formed is spread on the surface of a glass plate, and scraped into a thickness of 250um under normal temperature and pressure. liquid film. After 10 seconds, place the glass plate together with the liquid film in a constant temperature and humidity chamber at 50°C with a humidity of 80%, and take it out after 5 minutes to form a porous diaphragm.
[0038] The prepared porous diaphragm was soaked in deionized water for 24 hours, and then immersed in a solution of pyridine:water=1:3 (volume ratio) for 12 hours. Afterwards, the porous membrane was washed with deionized water, and immersed in 3mol / L sulfuric acid aqueous solution for 24 hours to obtain a porous composite membrane. The grafted pyridine group accounted for 20wt.% of the total mass of the porous composite membrane, and the cross section of the membrane with su...
Embodiment 2
[0044] 1g of chloromethyl polysulfone (the degree of chloromethylation is 135mmol / g) is blended with 1g of ordinary polysulfone, dissolved in 8g of DMAC, stirred for 24 hours, and the resulting polymer solution is spread on the surface of a glass plate and kept at room temperature. Press down and scrape into a liquid film with a thickness of 250um. After 10 seconds, place the glass plate together with the liquid film in a constant temperature and humidity chamber at 50°C with a humidity of 80%, and take it out after 5 minutes to form a porous diaphragm.
[0045] The prepared porous diaphragm was soaked in deionized water for 24 hours, and then immersed in a solution of pyridine:water=1:9 (volume ratio) for 24 hours. Afterwards, the porous membrane was washed with deionized water, and immersed in 3 mol / L sulfuric acid aqueous solution for 24 hours. The grafted pyridine group accounts for 20wt.% of the total mass of the porous composite membrane.
[0046] The prepared porous s...
Embodiment 3
[0048] 1g of bromomethylated polysulfone (the degree of bromomethylation is 100mmol / g) was stirred for 15 hours, and the formed polymer solution was spread on the surface of a glass plate, and then quickly immersed in 5L of water to solidify to form a porous diaphragm.
[0049] The prepared porous diaphragm was soaked in deionized water for 24 hours, and then immersed in a solution of imidazole:water=1:3 (volume ratio) for 24 hours. Afterwards, the porous membrane is washed with deionized water and immersed in 3 mol / L sulfuric acid aqueous solution for 24 hours to obtain an alkaline porous membrane containing imidazole groups. The grafted imidazole groups accounted for 15wt.% of the total mass of the porous composite membrane.
[0050] Utilize the prepared porous membrane to assemble a lithium-sulfur secondary battery, wherein the catalytic layer is activated carbon felt, the bipolar plate is a graphite plate, and the effective area of the membrane is 9 cm -2 , the current ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| porosity | aaaaa | aaaaa |
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com