Live vaccine heat-resistant protective agent and its preparation method and application
A heat-resistant protective agent and live vaccine technology, which is applied in antiviral agents, pharmaceutical formulas, medical preparations with non-active ingredients, etc., can solve the problems of much lower virus content, high cost, and long freeze-drying time. And the effect of simple preparation procedure, less drop in vaccine titer and stable potency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
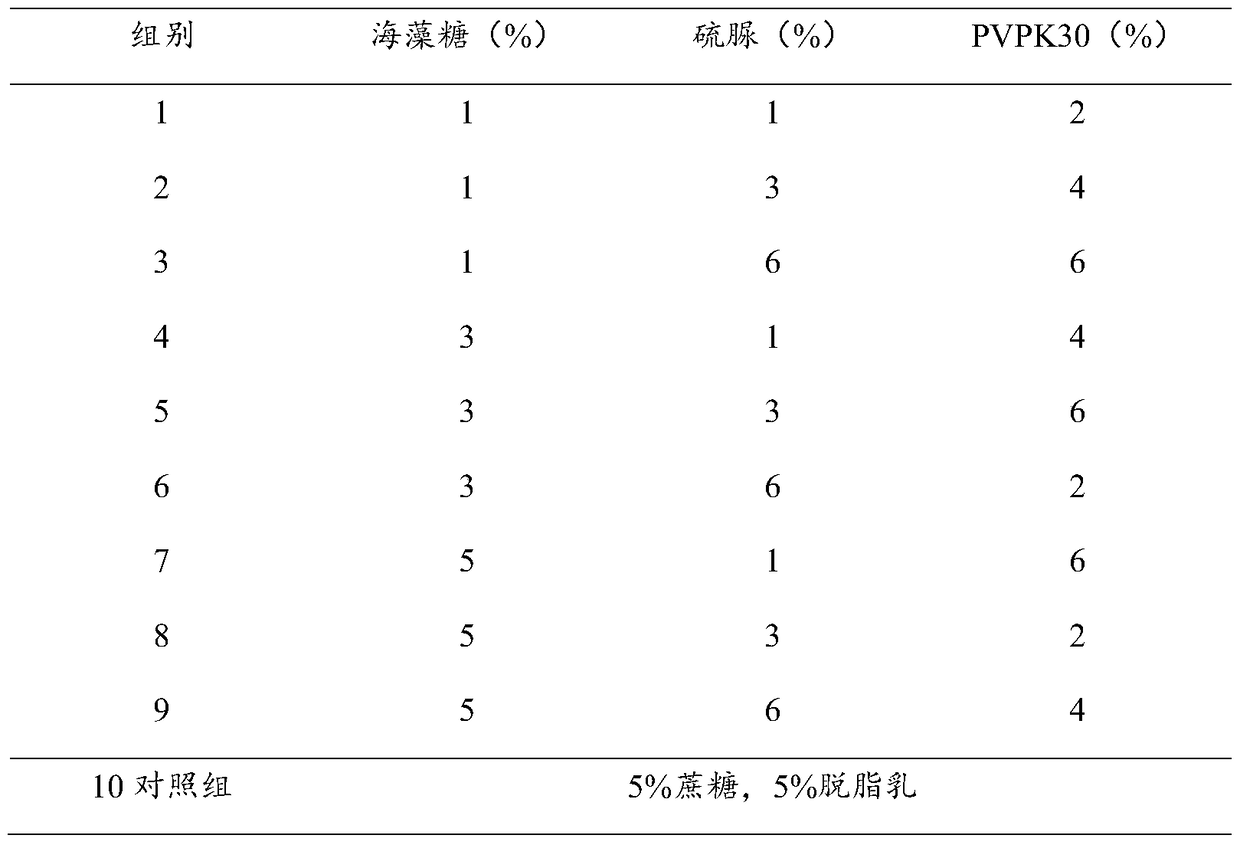
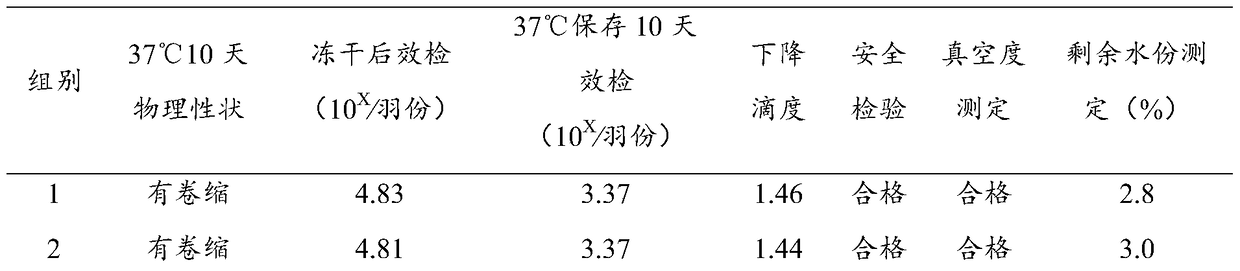
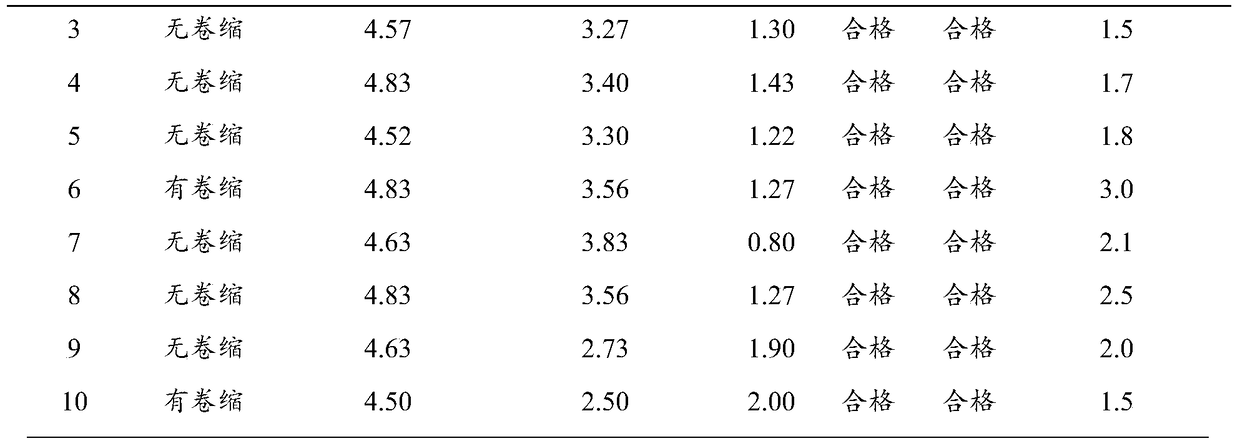
[0035] The preparation of embodiment 1 heat-resistant protective agent
[0036] Weigh each component according to the following proportions:
[0037] Trehalose: 5g;
[0038] Glucose: 5g;
[0039] Gelatin: 0.5g;
[0040] Thiourea: 1g;
[0041] PVPK30: 6g;
[0042] Add pure water to 100ml.
[0043] Mix the above solutions evenly, and sterilize at 121°C for 15 minutes to obtain.
Embodiment 2
[0044] The preparation of embodiment 2 heat-resistant protective agent
[0045] Weigh each component according to the following proportions:
[0046] Trehalose: 5g;
[0047] Glucose: 10g;
[0048] Gelatin: 2g;
[0049] Thiourea: 6g;
[0050] PVPK30: 6g;
[0051] Add pure water to 100ml.
[0052] Mix the above solutions evenly, and sterilize at 121°C for 15 minutes to obtain.
Embodiment 3
[0053] The preparation of embodiment 3 heat-resistant protective agent
[0054] Weigh each component according to the following proportions:
[0055] Trehalose: 5g;
[0056] Glucose: 10g;
[0057] Gelatin: 2g;
[0058] Thiourea: 1g;
[0059] PVPK30: 6g;
[0060] Add pure water to 100ml.
[0061] Mix the above solutions evenly, and sterilize at 121°C for 15 minutes to obtain.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com