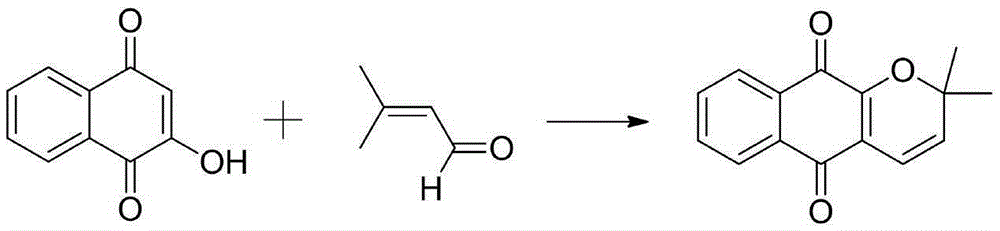
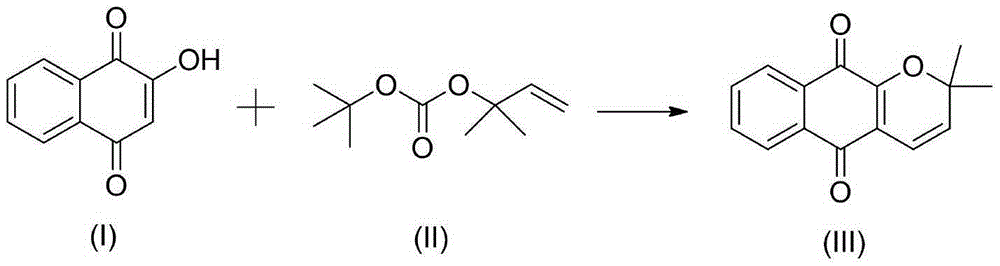
Catalyzed synthesis method of dehydrogenated alpha-lapachol
A synthesis method and compound technology, applied in the field of catalytic synthesis of dehydroα-lapachone, capable of solving problems such as low reaction yield, unfavorable industrial production, and narrow scope of application of substrates
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] In reactor, add 1mmol formula (I) compound, 2.3mmol potassium acetate and 28ml solvent acetic acid, continue to add 0.07mmol catalyst, 2.1mmol oxygenant Cu(OTf) under stirring 2 And 0.11mmol auxiliary agent, then add 4.1mmol formula (II) compound, stir and react at room temperature for 150min, after the reaction is completed, cool to room temperature, filter, wash with cold water successively, extract with ethyl acetate, wash with saturated saline solution, separate The organic phase was dried over anhydrous sodium sulfate, filtered, concentrated in vacuo, and then separated through a silica gel column (petroleum ether / ethyl acetate) to obtain the compound of formula (III) with a yield of 98.1%.
[0029] Among them, the catalyst is Pd(acac) with a molar ratio of 3:1 2 with CeCl 3 The mixture; auxiliary agent is 1-hexyl-3-methylimidazolium chloride salt.
Embodiment 2
[0031]
[0032] In reactor, add 1mmol formula (I) compound, 2.4mmol potassium acetate and 30ml solvent acetic acid, continue to add 0.08mmol catalyst, 2.2mmol oxidant Cu(OTf) under stirring 2 And 0.12mmol auxiliary agent, then add 4.2mmol formula (II) compound, stir and react at room temperature for 180min, after the reaction is completed, cool to room temperature, filter, wash with cold water successively, extract with ethyl acetate, wash with saturated saline solution, separate The organic phase was dried over anhydrous sodium sulfate, filtered, concentrated in vacuo, and then separated through a silica gel column (petroleum ether / ethyl acetate) to obtain the compound of formula (III) with a yield of 97.6%.
[0033] Among them, the catalyst is Pd(acac) with a molar ratio of 3:1 2 with CeCl 3 The mixture; auxiliary agent is 1-hexyl-3-methylimidazolium chloride salt.
Embodiment 3
[0035]
[0036] In reactor, add 1mmol formula (I) compound, 2.2mmol potassium acetate and 25ml solvent acetic acid, continue to add 0.06mmol catalyst, 2mmol oxidant Cu(OTf) under stirring 2 And 0.1mmol auxiliary agent, then add 4mmol formula (II) compound, stir and react at room temperature for 120min, after the reaction is completed, cool to room temperature, filter, wash with cold water successively, extract with ethyl acetate, wash with saturated saline solution, separate the organic The phase was dried over anhydrous sodium sulfate, filtered, concentrated in vacuo, and then separated through a silica gel column (petroleum ether / ethyl acetate) to obtain the compound of formula (III) with a yield of 97.5%.
[0037] Among them, the catalyst is Pd(acac) with a molar ratio of 3:1 2 with CeCl 3 The mixture; auxiliary agent is 1-hexyl-3-methylimidazolium chloride salt.
[0038] The present invention has confirmed and characterized the structures of the target products of all...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com