Cow inhibin alpha subunit mature peptide gene, and recombinant expression protein method
A gene recombination and inhibin technology, applied in genetic engineering, plant gene improvement, recombinant DNA technology, etc., can solve the problems of increasing the difficulty and cost of the test, and the lack of natural activity of the protein, so as to reduce the technical difficulty and cost, and achieve good reaction. Sexuality, beneficial to industrialization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
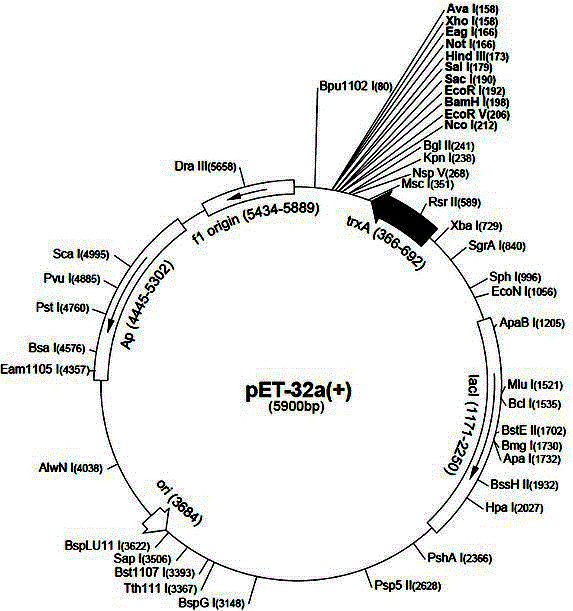
[0115] Embodiment 1, bovine InhibinpET32a(+)-matpeptide recombinant vector construction
[0116] 1. Primer design
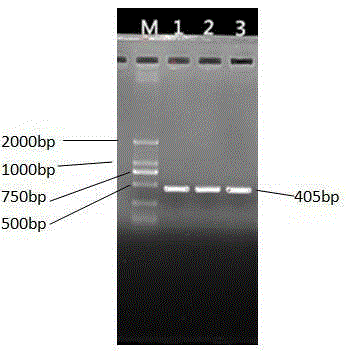
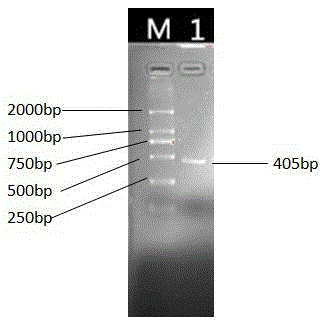
[0117] Referring to the complete sequence of Bostaurusinhibin, alpha (INHA), NM_174094.4 gene nucleotide matpeptide published on GenBank, a pair of upstream and downstream primers were designed using the primer design software Primer5.0, and 405 nucleotides at the 3′ end were cut out. The sequence of SEQ ID NO: 1 in the sequence listing was obtained.
[0118] Upstream primer P1: 5'-cgggatcccgctccacgcccccactg-3' (26bp) (SEQ ID NO: 3)
[0119] Downstream primer P2: 5'-ccgctcgagaatcccttagatgcaagcaca-3' (30bp) (SEQ ID NO: 4).
[0120] 2. RT-PCR amplification of bovine inhibin matpeptide fragment
[0121] (1) Extraction of bovine ovarian follicle RNA
[0122] ①The ovaries obtained from the slaughterhouse were cut with scissors to cut out the surrounding tissues of the follicles, quick-frozen in liquid nitrogen, and stored at -80°C for later use.
[0123] Soak all...
Embodiment 2
[0178] Embodiment 2: Expression of bovine pET32a(+)-matpeptide-E.coliBL21(DE3) genetic engineering bacteria
[0179] 1. Transformation of recombinant plasmids into host bacteria;
[0180] The recombinant plasmid sequenced correctly in Example 1 was transformed according to the transformation steps, and the LB liquid medium, LB solid medium and AMP storage solution required for transformation were formulated as follows:
[0181] ①LB liquid culture medium
[0182] Tryptone………………………… 1.0g
[0183] YeastExtract……………………0.5g
[0184] NaCl……………………………… 1.0g
[0185] Make up to 100ml with distilled water
[0186] ②LB solid medium
[0187] Tryptone………………………… 1.0g
[0188] Yeast Extract………………… 0.5g
[0189] NaCl……………………………… 1.0g
[0190] Powderedagar……………………1.5g
[0191] Make up to 100ml with distilled water
[0192] ③AMP storage solution
[0193] Weigh 0.5g of Amp and dissolve it in 5ml of deionized water, filter and sterilize through a 0.22um filter, aliquot and store at -20...
Embodiment 3
[0216] Example 3. Application and Immunological Detection of Bovine Inhibin α Subunit Mature Peptide Recombinant Protein of the Present Invention
[0217] In order to obtain the anti-inhibin α subunit rabbit polyclonal antibody with higher titer and increase the ovulation rate of animals, the purified bovine inhibin α subunit recombinant protein of the present invention obtained in Example 2 was used to actively immunize rabbits, blood was collected from the heart and The serum was separated, and the antibody titer was detected by indirect ELISA method to determine whether the corresponding antibody was produced.
[0218] Among them, the rabbit anti-bovine inhibin α subunit polyclonal antibody serum is prepared by the following method:
[0219] The protein obtained in Example 2 was used as an antigen to actively immunize rabbits to prepare polyclonal antiserum.
[0220] 1. Preparation of antigen in Freund's adjuvant by emulsification method
[0221] Boil a glass syringe, a 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com