Fully aromatic phenolic amine copolymer containing multifunctional groups and its preparation method and application
An aromatic phenolamine copolymer, multifunctional technology, applied in chemical instruments and methods, other chemical processes, adsorption water/sewage treatment, etc. See reports and other issues to achieve the effect of improving the degree of compact packing, saving raw materials, and good solvent resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
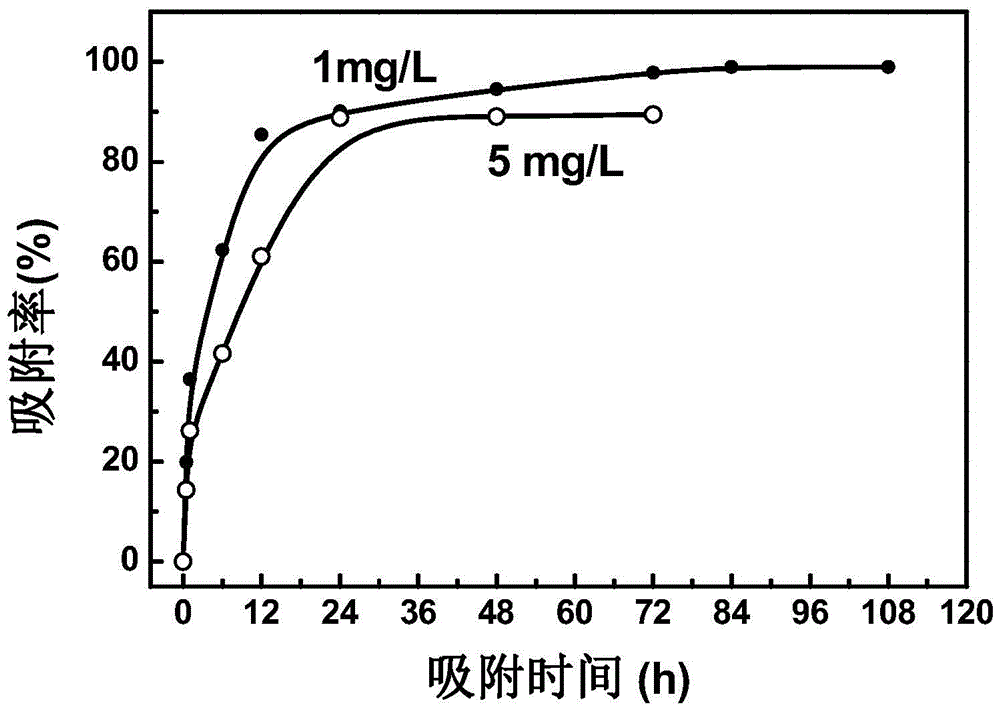
Examples
Embodiment 1
[0046] Accurately weigh 2.054g of m-phenylenediamine and 0.110g of hydroquinone into a 50mL Erlenmeyer flask, add 20ml of deionized water to ultrasonically dissolve them, mix the monomer solutions and place them in a 0°C water bath and stir , equilibrate for 30min, accurately weigh 13.70g of persulfate and dissolve it in 150ml of deionized water, and maintain it in a water bath at 0°C, add the persulfate solution dropwise to the above mixed monomer solution at a rate of 1 drop / 3 seconds middle. After the addition was complete, the stirring reaction was continued until 6h. Track the potential, pH and temperature changes of the reaction solution throughout the process. After the reaction is over, pour the reaction solution into a centrifuge tube for centrifugation. After the polymer settles, use a dropper to absorb the upper layer liquid and wash it with deionized water. After dispersing, wash it again; repeat the above operation until the upper layer liquid in the centrifuge t...
Embodiment 2~6
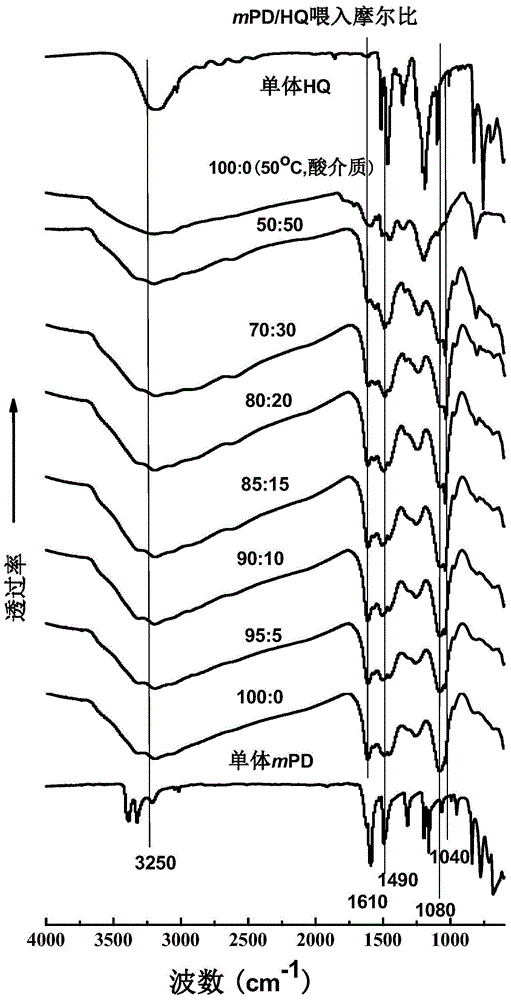
[0048] According to the method of embodiment 1, the molar ratio (total molar number is 0.02mol) of changing m-phenylenediamine and hydroquinone is respectively 90:10, 85:15, 80:20, 70:30, 50:50, The doped m-phenylenediamine-hydroquinone copolymers were prepared, and the apparent yields of the copolymers were 88.2%, 90.1%, 91.3%, 95.6%, and 87.2%, respectively. The resulting doped copolymers were all transferred to ammonia water and stirred for 6 hours, dried and weighed after centrifugation to obtain de-doped powdered copolymer products, and the calculated yields were 64.9%, 66.0%, 68.4%, respectively. 72.1%, 55.2%.
Embodiment 7
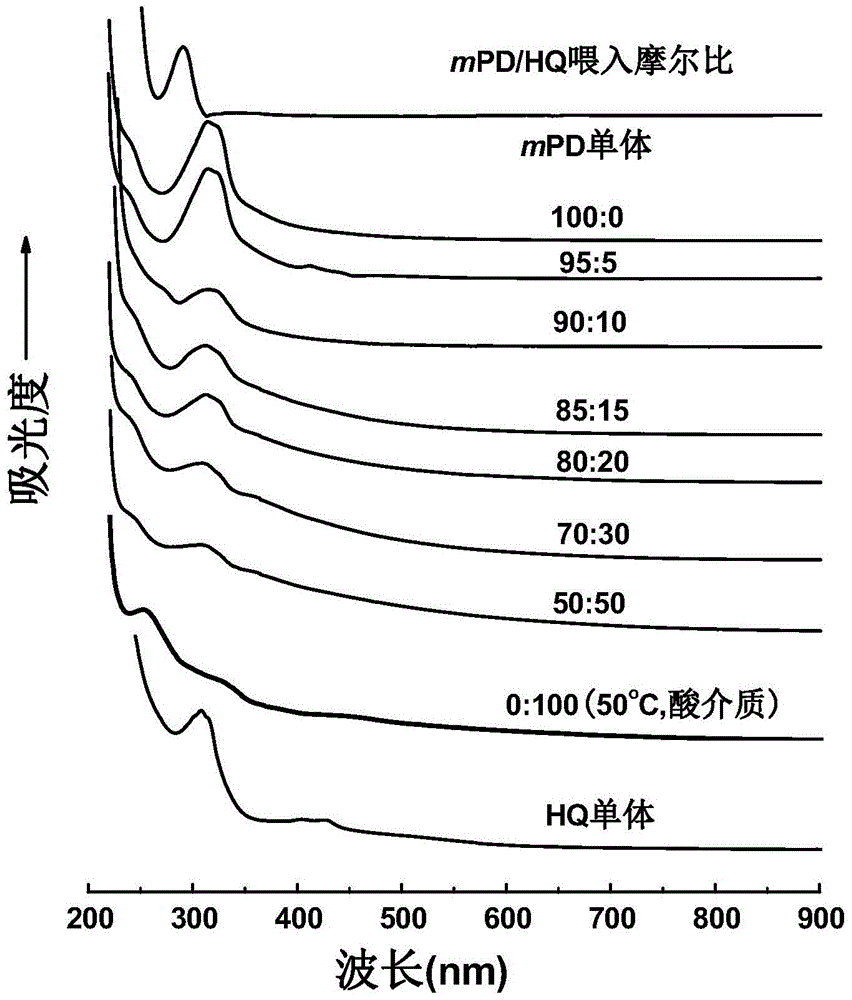
[0058] UV testing of m-phenylenediamine hydroquinone copolymers. About 2 mg of the copolymers obtained in Examples 1 to 6 and the polym-phenylenediamine and polyhydroquinone obtained in Comparative Examples 1 and 3 were respectively placed in 1M NaOH solution. The degree of solubility of the copolymers was different. The solution of copolymerization product carries out ultraviolet test respectively subsequently, and ultraviolet absorption spectrum is as follows figure 1 shown. There are obvious differences between the peaks of the polymer and the two monomers. Polyhydroquinone has two obvious shoulder peaks, and it has a relatively strong tailing absorption peak in the high wavelength region, which shows that polyhydroquinone has A wide range of planar conjugate structures. Poly-m-phenylenediamine and copolymers have an obvious absorption peak at 300nm, which is caused by the π-π transition. Compared with the absorption peak of the monomer, it moves a certain distance to the...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com