Preparation method for optically-pure citronellol
A citronellol and optical technology, applied in the field of preparation of optically pure citronellol, can solve the problems of no cost advantage, high equipment investment, complicated process and the like, and achieve the effect of improving chemical selectivity and stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
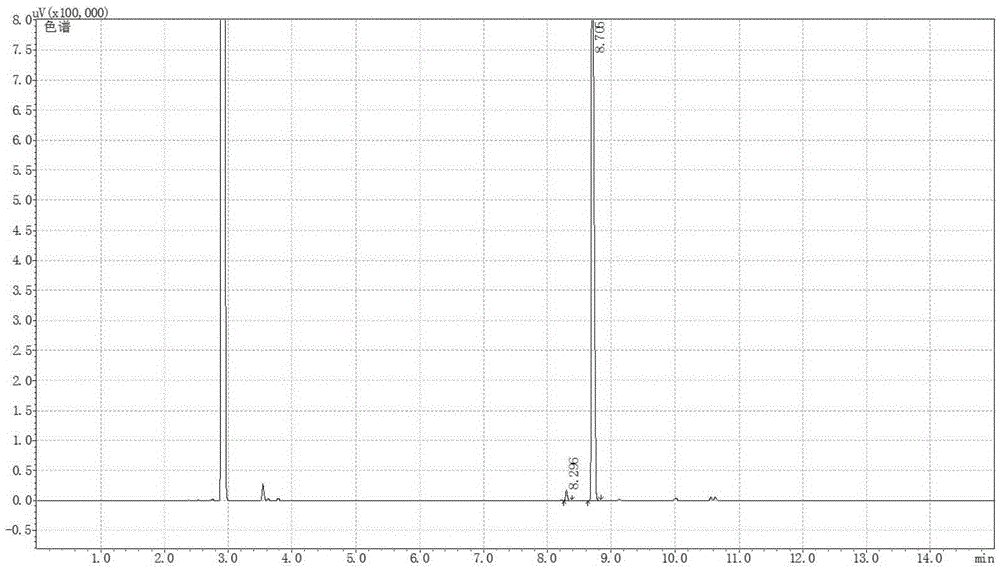
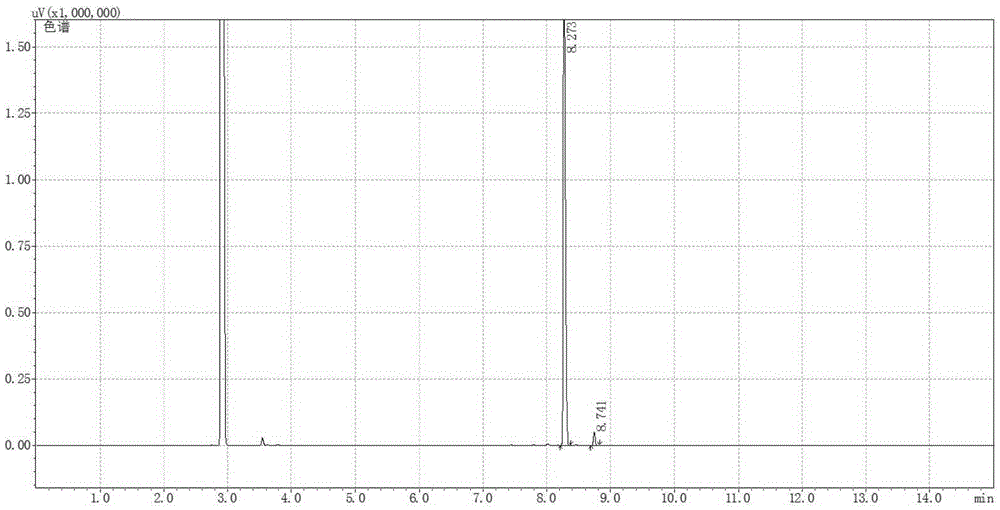
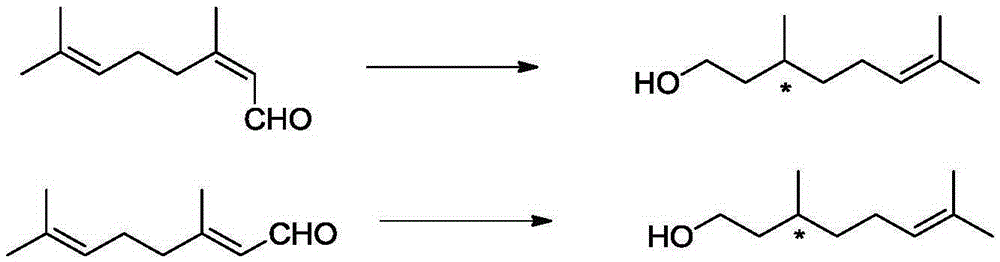
[0064] 41.4mg1,1'-Bis[(2R,5R)-dimethylphospholane-1-yl]-ferrocene, 40.6mgRh(cod) 2 BF 4 , 16.58mg of potassium iodide was dissolved in 40mL of methanol, and transferred to a 100mL autoclave, 15.22g of neral (neral / geranial=99.1:0.9(mol), substrate / catalyst=1000(mol)) Inject into the autoclave, and adjust the pressure to 50 bar after passing through hydrogen to replace the gas in the autoclave three times. Stirring was started, and after reacting at 40° C. for 10 h, the conversion rate was measured by gas chromatography to be 99.9%, the product was L-citronellol, the yield was 99.8%, and the optical purity was 99ee%.
Embodiment 2
[0066] 41.4mg1,1'-Bis[(2R,5R)-dimethylphospholane-1-yl]-ferrocene, 40.6mgRh(cod) 2 BF 4 16.58 mg of potassium iodide was dissolved in 130 mL of methanol, and transferred to a 200 mL autoclave, with 15.22 g of neral (the ratio of neral / geranial isomers was 95:5 (mol), and the ratio of substrate / catalyst = 1000 (mol)) was injected into the autoclave, and the pressure in the autoclave was adjusted to 50 bar after being introduced into the hydrogen for three times. Stirring was started, and after reacting at 40° C. for 10 h, the conversion rate was measured by gas chromatography to be 99.9%, the product was L-citronellol, the yield was 99.8%, and the optical purity was 89ee%.
Embodiment 3
[0068] 41.4mg1,1'-Bis[(2S,5S)-dimethylphospholane-1-yl]-ferrocene, 40.6mgRh(cod) 2 BF 4 82.9 mg of potassium iodide was dissolved in 9.7 mL of methanol, and transferred to a 50 mL autoclave, 7.61 g of neral (the ratio of neral / geranial double bond isomers was 99.1:0.9 (mol), the substrate / catalyst ratio=500 (mol)) was injected into the autoclave, and the pressure was adjusted to 60 bar after passing through the hydrogen displacement autoclave for three times. Stirring was started, and after reacting at 40°C for 12 hours, the conversion rate was measured by gas chromatography to be 99.8%, the product was d-citronellol, the yield was 99.7%, and the optical purity was 90ee%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com