Preparing method for pirfenidone
A technology of pirfenidone and pentenenitrile, applied in the field of preparation of pirfenidone, can solve the problems of 2-amino-5-picoline high price, unsatisfactory yield, complicated operation, etc. Industrialized production application, reduced production cost, good effect of reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
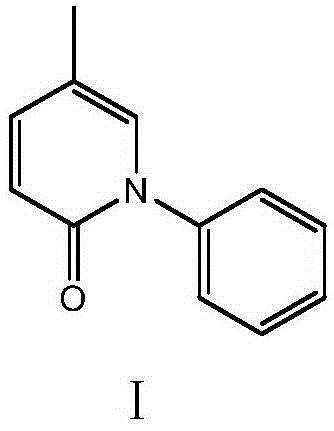
[0032] Embodiment 1: the preparation of pirfenidone (I)
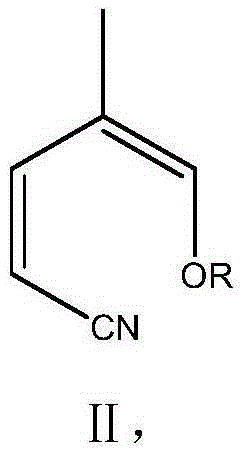
[0033] In the 500 milliliter four-neck flask that is connected with stirring, thermometer and distillation device, add 150 grams of N,N-dimethylformamide, 24.3 grams (0.3 moles) of 2-pentenenitrile, 0.8 grams of toluenesulfonic acid, 42.5 g (0.4 mol) of trimethyl orthoformate was stirred and reacted at 90 to 100° C. for 4 hours, while recovering the produced methanol. Cool to 80 to 85°C, add 30.6 g (0.33 mole) of aniline, stir and react at 90 to 95°C for 3 hours, while recovering the produced methanol. Cool to 20°C, add 250 grams of water, stir at 20°C for 2 hours for hydrolysis reaction, the imino group is hydrolyzed to form a carbonyl group, and pirfenidone is generated; filter, and the filter cake is recrystallized with 200 grams of isopropanol to obtain 51.5 grams of white solid pirfenidone Nitone (I), the yield is 92.7%, and the liquid phase purity is 99.9%.
Embodiment 2
[0034] Embodiment 2: the preparation of pirfenidone (I)
[0035] In the 500 milliliter four-neck flask that is connected with stirring, thermometer and distillation device, add 150 grams of N,N-dimethylformamide, 24.3 grams (0.3 moles) of 2-pentenenitrile, 0.7 grams of toluenesulfonic acid, 59.5 g (0.4 mol) of triethyl orthoformate was reacted with stirring at 100 to 105° C. for 4 hours, and the ethanol produced was recovered at the same time. Cool to 80-85°C, add 30.5 g (0.33 moles) of aniline, raise the temperature to 100-105°C and stir for 3 hours, while recovering the ethanol produced. Cooled to 20°C, added 250 grams of water, hydrolyzed at 20°C for 2 hours, filtered, and the filter cake was recrystallized with 200 grams of isopropanol to obtain 52.0 grams of white solid pirfenidone (I), yield 93.6%, liquid phase 99.9% pure.
Embodiment 3
[0036] Embodiment 3: the preparation of pirfenidone (I)
[0037] In a 500 ml four-neck flask connected with a stirring, thermometer and distillation device, add 150 g of N,N-dimethylformamide, 24.3 g (0.3 moles) of 2-pentenenitrile, 0.6 g of 98% concentrated sulfuric acid, 42.0 gram (0.4 moles) of trimethyl orthoformate, reacted with stirring at 95 to 105° C. for 4 hours, and simultaneously recovered the produced methanol. Cool to 80 to 85°C, add 29.0 g (0.31 mole) of aniline, stir and react at 95 to 105°C for 3 hours, while recovering the produced methanol. Cool to 20°C, add 250 grams of water, stir and hydrolyze for 2 hours at 20°C, filter, and recrystallize the filter cake with 200 grams of isopropanol to obtain 51.0 grams of white solid pirfenidone (I), with a yield of 91.8%. Phase purity 99.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com