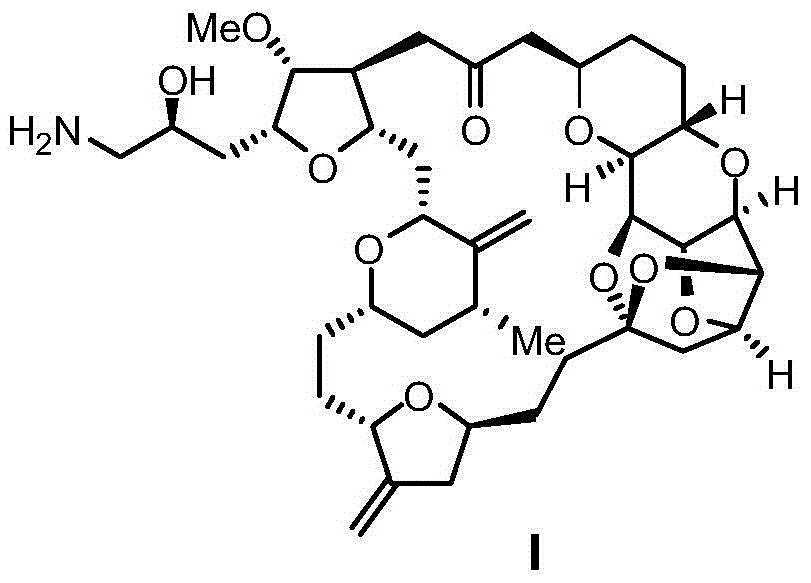
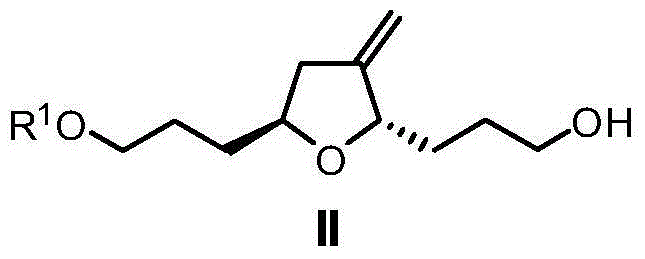
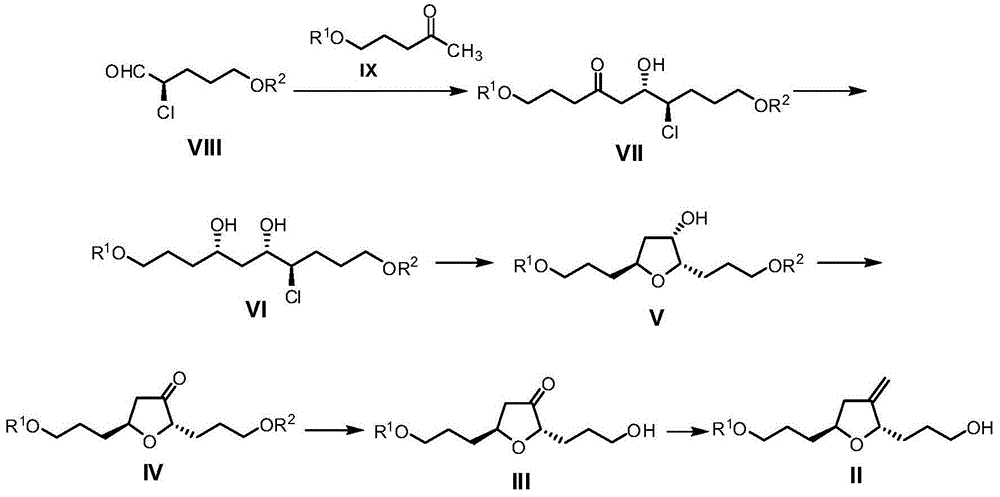
Preparation method of eribulin intermediate
A technology of compound and hydroxyl protecting group, applied in the field of preparation of eribulin intermediates, can solve the problems of difficult to realize scale-up production, complex, difficult to scale-up production and application, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Embodiment 1: preparation compound VIIa
[0083] Compound IXa (13.1 g, prepared according to literature Tetrahedron Lett. 2001, 42, 9233) was dissolved in tetrahydrofuran (240 mL), cooled to -10 ° C, dropwise added LDA tetrahydrofuran solution (46 mL, 1.0 M), at -10 ° C After reacting at low temperature for 2 hours, add compound VIIIa (6.5g, prepared according to the literature Angew.Chem.Intl.Ed.2009, 48, 5121), and react at -10°C for 2 hours, add saturated aqueous ammonium chloride (56mL ) to quench the reaction, add 200mL ethyl acetate, separate the organic phase, dry the organic phase with anhydrous sodium sulfate, concentrate and purify by column chromatography to obtain compound VIIa (14.2g).
[0084] 1 HNMR (CDCl 3 ,400MHz)δ=7.67-7.62(m,4H),7.46-7.32(m,11H),4.50(s,2H),4.12-4.07(m,1H),3.94-3.88(m,1H),3.67( t,J=6.0Hz,2H),3.54-3.48(m,2H),3.28(d,J=5.0Hz,0.6H),2.83-2.68(m,2H),2.58(t,J=7.4Hz, 2H), 2.08-1.69(m, 7H), 1.05(s, 9H).
Embodiment 2
[0085] Embodiment 2: Preparation of compound VIa
[0086]Compound VIIa (12.2g) was dissolved in tetrahydrofuran (330mL), cooled to -30°C, DIBAL-H hexane solution (66mL, 1.0M) was added dropwise, reacted at -30°C for 5 hours, and 8mL of methanol was added The reaction was quenched with 160 mL saturated potassium sodium tartrate aqueous solution, 300 mL ethyl acetate was added, the organic phase was separated, the organic phase was dried over anhydrous sodium sulfate, concentrated and purified by column chromatography to obtain compound VIa (9.6 g).
[0087] 1 HNMR (CDCl 3 ,400MHz)δ=7.68-7.64(m,4H),7.46-7.32(m,11H),4.51(s,2H),4.10-4.07(m,1H),3.97-3.86(m,3H),3.73- 3.67 (m, 2H), 3.55-3.49 (m, 2H), 1.05 (s, 9H).
Embodiment 3
[0088] Embodiment 3: preparation compound Va
[0089] Dissolve compound VIa (8.0g) in tetrahydrofuran (300mL), cool to 0°C, add silver oxide (3.2g) and silver trifluoromethanesulfonate (3.5g), react at 20°C for 18 hours, add Saturated aqueous sodium bicarbonate solution was used to quench the reaction, and 200 mL of ethyl acetate was added to separate the organic phase. The organic phase was dried with anhydrous sodium sulfate, concentrated and purified by column chromatography to obtain compound Va (6.9 g).
[0090] 1 HNMR (CDCl 3 ,400MHz)δ=7.68-7.64(m,4H),7.42-7.32(m,11H),4.51(s,1.6H),4.50(s,0.4H),4.25-4.15(m,1.6H),4.05 -3.92(m,0.8H),3.79-3.74(td,J=2.9,6.9Hz,1H),3.70-3.65(m,2H),3.56-3.47(m,2H),2.09-1.99(m,2H ), 1.04(s,9H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com