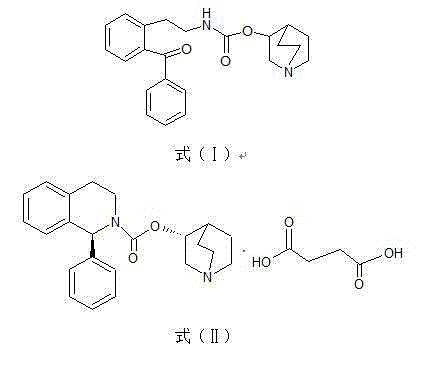
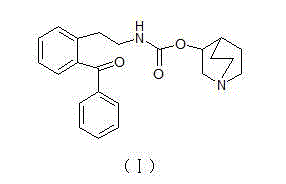
Preparation method for impurity of Solifenacin succinate
A solifenacin succinate impurity technology, which is applied in the field of preparation of solifenacin succinate impurity [Formula (I)], can solve the problems of compound preparation methods without literature reports, etc., and achieve high product purity and excellent reaction operation simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Get 5g of solifenacin succinate, stir and dissolve in the mixed solvent of 50ml of water and 5ml of acetone. Add 5.6g of potassium permanganate, stir at 20-25°C for 8h, and let stand overnight. The conversion rate of [2-(2-benzoyl-phenyl)-ethyl]-carbamic acid 1-aza-bicyclo[2.2.2]oct-3-yl ester was 45% by HPLC the next day. Filter, add 25ml of ethyl acetate for layering, adjust the pH to 9~10 with saturated potassium carbonate solution, and extract the aqueous layer with 25ml×2 ethyl acetate. The organic layers were combined and dried over anhydrous magnesium sulfate for 2 h. The desiccant was filtered off and concentrated under reduced pressure to obtain 3.4 g of a yellow oil. Separation by column chromatography (dichloromethane / methanol=15 / 1), TLC (dichloromethane / methanol=10 / 1) to monitor the separation process, collect the eluent, and concentrate under reduced pressure to dryness to obtain a white solid [2-(2 -Benzoyl-phenyl)-ethyl]-carbamic acid 1-aza-bicyclo[2.2...
Embodiment 2
[0030] Get 10g of solifenacin succinate, stir and dissolve in a mixed solvent of 100ml of water and 10ml of acetone. Add 11.2g of potassium permanganate, stir at 20-25°C for 8h, and leave overnight. The conversion rate of [2-(2-benzoyl-phenyl)-ethyl]-carbamic acid 1-aza-bicyclo[2.2.2]oct-3-yl ester was 54.6% by HPLC the next day. Filter, add 50ml of dichloromethane and 5% sodium hydroxide solution to adjust the pH to 9~10, separate the water layer, extract the water layer with 50ml×2 dichloromethane, combine the organic layers and dry with anhydrous magnesium sulfate for 2h. The desiccant was filtered off and concentrated under reduced pressure to obtain 6.5 g of a yellow oil. Separation by column chromatography (dichloromethane / methanol=15 / 1), TLC (dichloromethane / methanol=10 / 1) to monitor the separation process, collect the eluent, and concentrate under reduced pressure to dryness to obtain a white solid [2-(2 -Benzoyl-phenyl)-ethyl]-carbamic acid 1-aza-bicyclo[2.2.2]oct-3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com