A method for comprehensive recycling of lithium-containing aluminum electrolyte
An electrolyte, lithium-aluminum technology, applied in the field of comprehensive recycling and utilization of lithium-aluminum electrolytes, can solve the problems of many accompanying impurity ions, difficult to high-end fields, unable to release production capacity, etc., to reduce production costs, product market competitiveness is strong, The effect of saving fluorite
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
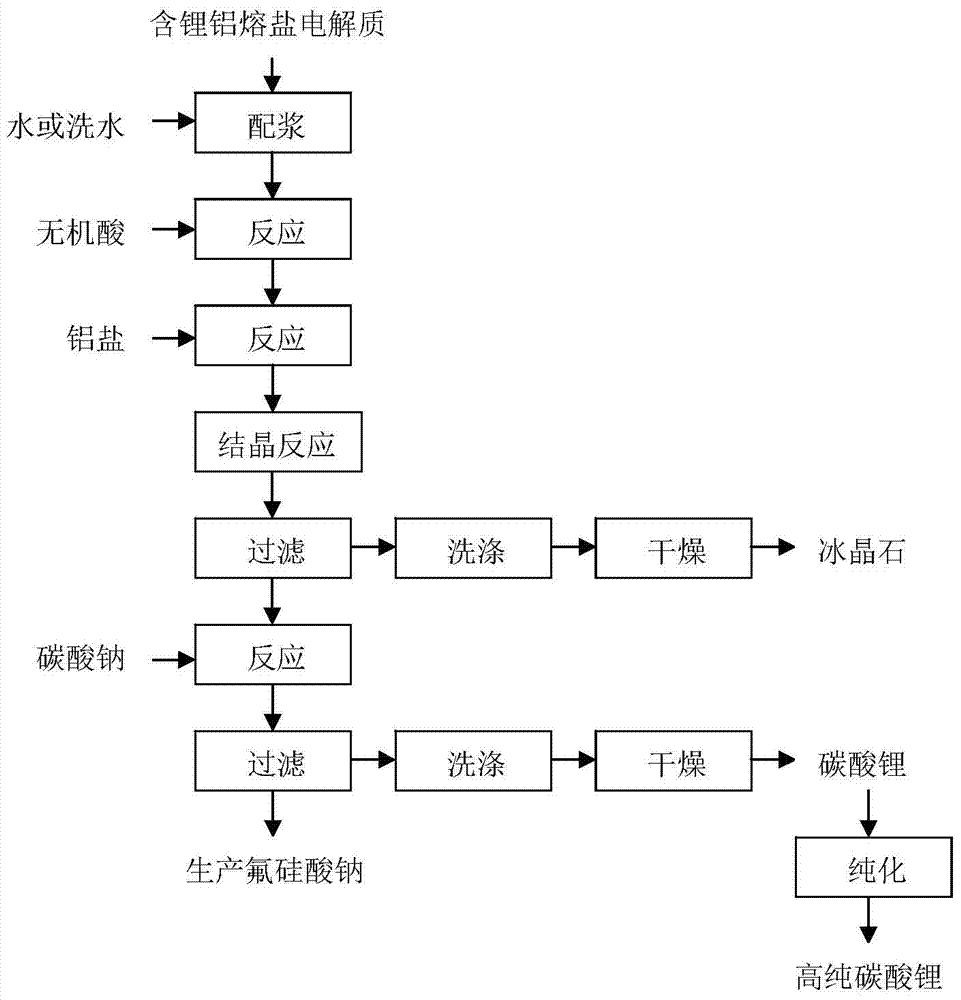
[0030] The method for the comprehensive recycling of the lithium-containing aluminum electrolyte of this embodiment, the process flow is as follows figure 1 shown, including the following steps:
[0031] 1) Add 1000g of aluminum electrolyte containing 3wt% lithium fluoride and 85wt% cryolite and 3000g pure water into the reaction kettle and mix them; add 30wt% hydrochloric acid to adjust the pH of the system to 1, and stir and react at 10°C for 3h to obtain slurry;
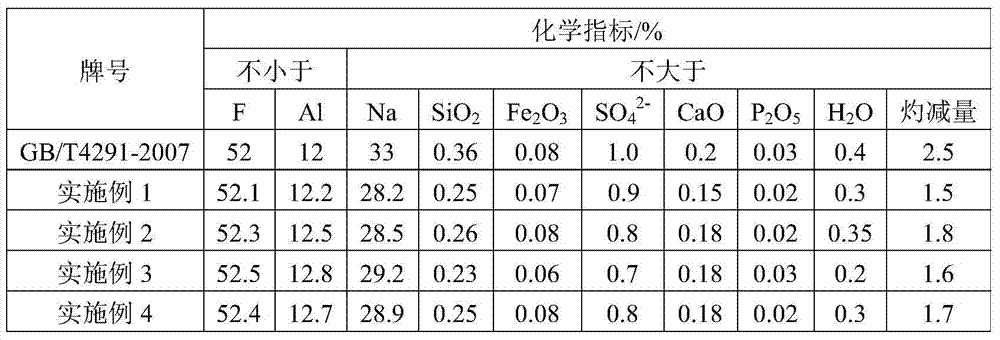
[0032] 2) According to lithium fluoride: aluminum trichloride molar ratio 3:1, add 54g aluminum trichloride (purity is 95wt%) in the slurry obtained in step 1), heat up to 95 ℃ and react for 2h to convert lithium fluoride into Lithium chloride and aluminum fluoride are filtered, washed, and dried to obtain 878g of cryolite product; the washing water is neutralized with lime and discharged;
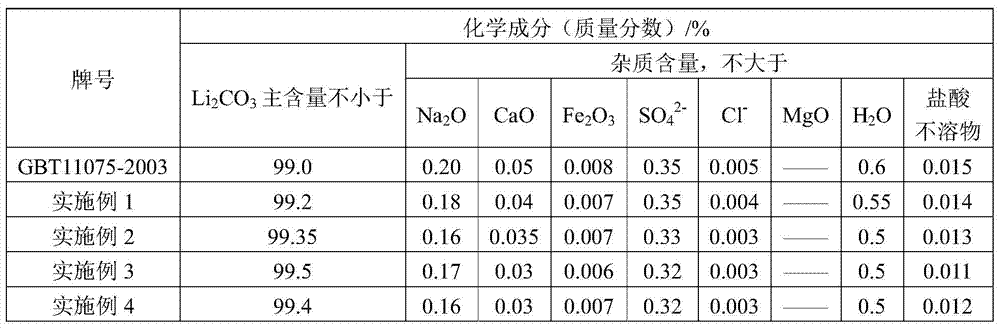
[0033] 3) add 63g sodium carbonate (purity is 98wt%) in step 2) gained filtrate by lithium chloride and sodium carbonate m...
Embodiment 2
[0036] The method for the comprehensive recycling of the lithium-containing aluminum electrolyte of this embodiment, the process flow is as follows figure 1 shown, including the following steps:
[0037] 1) Add 1000 g of aluminum electrolyte containing 10wt% lithium fluoride, 80wt% cryolite and 2000g pure water into the reaction kettle and mix; add 98wt% sulfuric acid to adjust the pH of the system to 0, and stir and react at 40°C for 2h to obtain slurry;
[0038]2) Add 231g of aluminum sulfate (purity: 95wt%) to the slurry obtained in step 1) according to the molar ratio of lithium fluoride: aluminum sulfate 6:1, heat up to 95°C for 3 hours to convert lithium fluoride into lithium sulfate and fluorine Aluminum, filtered, washed, and dried to obtain 900g of cryolite product; the washing water is neutralized with lime and discharged;
[0039] 3) add 205g sodium carbonate (purity is 98wt%) in step 2) gained filtrate by lithium sulfate and sodium carbonate mol ratio 1:1, carry ...
Embodiment 3
[0042] The method for the comprehensive recycling of the lithium-containing aluminum electrolyte of this embodiment, the process flow is as follows figure 1 shown, including the following steps:
[0043] 1) Add 1000g of aluminum electrolyte containing 15wt% lithium fluoride, 82wt% cryolite and 6000g pure water into the reaction kettle and mix them; add 98wt% nitric acid to adjust the pH of the system to 1.5, and stir and react at 45°C for 2h to obtain slurry;
[0044] 2) According to lithium fluoride: aluminum nitrate molar ratio 3:1, add 410g aluminum nitrate (purity is 98wt%) in the slurry obtained in step 1), heat up to 95 ℃ and react for 2.5h to convert lithium fluoride into lithium nitrate and Aluminum fluoride, filtered, washed, and dried to obtain 975g of cryolite product; the washing water was neutralized with lime and discharged;
[0045] 3) add 313g sodium carbonate (purity is 98wt%) in step 2) gained filtrate by lithium nitrate and sodium carbonate mol ratio 2:1, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com