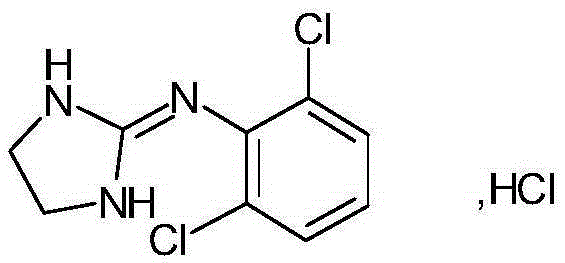
Clonidine adhesive patch and preparation method thereof
A technology of clonidine and cola, which is applied in the field of pharmaceutical preparations in the medical field, can solve the problems of foreign body sensation, no clonidine patch, and poor effect of long-term controlled release, and achieve the effect of preventing sudden release
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
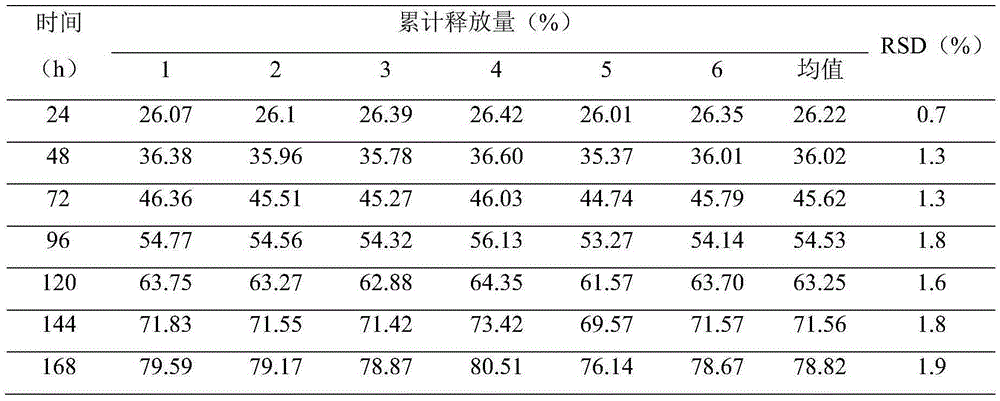
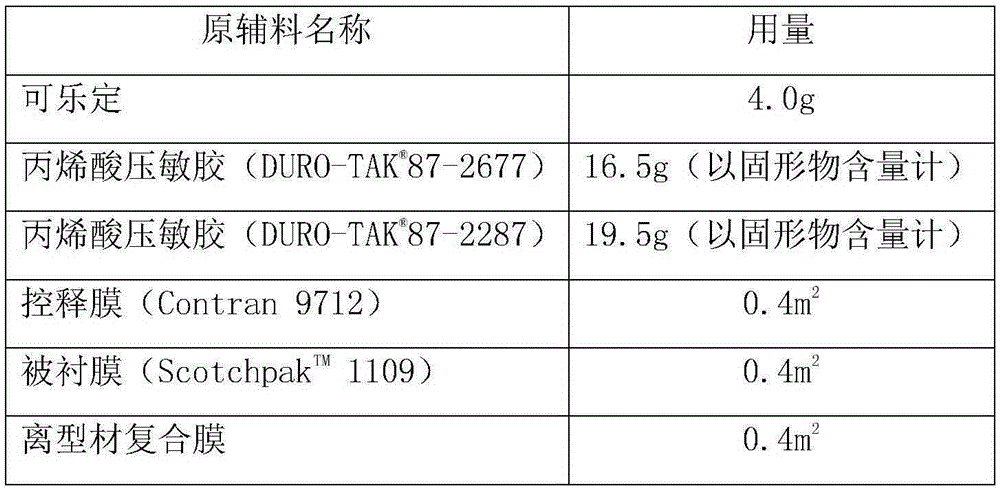
[0087] 1. Composition of clonidine patch (1000 patches):
[0088]
[0089] 2. the preparation method of clonidine patch, comprises the following steps:
[0090] (1) Clonidine layer preparation: take the clonidine of formula quantity, put 80% quantity in ball mill (100ml tank) and mix according to the ratio of 1:1-8 (W:V) with B, the size grinding ball ratio is (10-30): (20-50), rotating speed is 200~400rpm, grinds 2~12 hours, obtains the paste that clonidine content is 20% (wt), and this clonidine is divided into the clonidine of 20% amount Micropowder (0.8g) and 80% clonidine paste (3.2g);
[0091] (2), the clonidine micropowder of above-mentioned 20% amount is mixed with acrylic pressure-sensitive adhesive 87-2287, is coated on the release material composite film, is dried, and is pressed and coated into clonidine composite film together with controlled-release film; Wherein drying It is a five-section temperature-controlled drying process, that is, the drying process i...
Embodiment 2
[0096] 1. Composition of clonidine patch (1000 patches):
[0097]
[0098] 2. the preparation method of clonidine patch, comprises the following steps:
[0099] (1) Clonidine layer preparation: Weigh the clonidine of the formula amount, put 80% of the amount in a ball mill (100ml tank) and mix it with n-heptane according to the ratio of 1:1-8 (W:V), the size of the grinding ball Ratio is (10-30):(20-50), rotating speed is 200~400rpm, grinds 2~12 hours, obtains the paste that clonidine content is 20% (wt), and this clonidine is divided into 20% amount Clonidine micropowder (0.8g) and the clonidine paste (3.2g) of 80% amount;
[0100] (2), the clonidine micropowder of above-mentioned 20% amount is mixed with acrylic pressure-sensitive adhesive 87-2287, is coated on the release material composite film, is dried, and is pressed into clonidine composite film together with controlled-release film; Wherein drying is Five-section temperature control, that is, the drying process i...
Embodiment 3
[0104] 1. Composition of clonidine patch (1000 patches):
[0105]
[0106] 2. the preparation method of clonidine patch, comprises the following steps:
[0107](1) Clonidine layer preparation: Weigh 80% of the clonidine in the formula and place it in a ball mill (100ml tank) and mix it with n-heptane at a ratio of 1:1-8 (W:V). For (10-30): (20-50), rotating speed is 200~400rpm, grinds 2~12 hours, obtains the paste that clonidine content is 20% (wt), and this clonidine is divided into the cola of 20% amount Clonidine paste (3.2g) of clonidine powder (0.8g) and 80% amount;
[0108] (2), the clonidine micropowder of above-mentioned 20% amount is mixed with acrylic pressure-sensitive adhesive 87-2287, is coated on the release material composite film, is dried, and is pressed and coated into clonidine composite film together with controlled-release film; Wherein drying It is a five-section temperature-controlled drying process, that is, the drying process is divided into five ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com