Catalyst for synthesizing gas light hydrocarbon and preparation method of catalyst
A technology for synthesis gas and light hydrocarbons, applied in chemical instruments and methods, physical/chemical process catalysts, metal/metal oxides/metal hydroxide catalysts, etc. The selectivity is not high enough, the product is not concentrated enough, etc., to achieve good technical effects, excellent catalytic effects, and improved activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
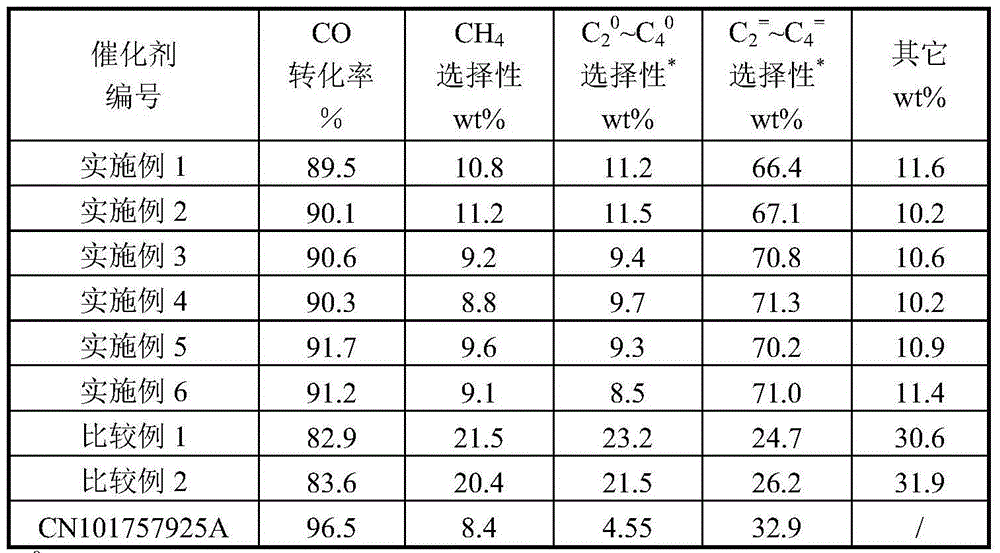
Examples
Embodiment 1
[0059] Take 587.8 grams of ferric nitrate, add 1000 g of water to dissolve, obtain material I, take 0.4 g of lanthanum nitrate and add 10 g of water to dissolve, obtain material II, take 25.5 grams of 50% manganese nitrate and 21.64 grams of zinc nitrate in the same container, add 100 g of water , stirring and dissolving to obtain material III.
[0060]Materials I, II, and III are mixed, and 312.5 grams of 40% (weight) aluminum sol materials are added under stirring, then 50 g of a solution containing 0.1 gram of KOH is added, and the pH value of the above-mentioned slurry is adjusted with ammonia water to make the pH of the mixed slurry = 6.0, after sufficient stirring, the prepared slurry is carried out in a spray dryer according to the usual method to form microspheres, and finally the internal diameter is 89 mm, and the length is 1700 mm (φ89 × 1700 mm) in a rotary roaster. Calcined at 500°C for 2.0 hours, the composition of the prepared catalyst is:
[0061] 50 wt% Fe 1...
Embodiment 2
[0063] Take 587.7 grams of ferric nitrate, add 1000 g of water to dissolve, and obtain material I, take 0.4 g of lanthanum nitrate and add 10 g of water to dissolve, obtain material II, take 25.5 grams of 50% manganese nitrate and 21.63 grams of zinc nitrate in the same container, add 100 g of water , stirring and dissolving to obtain material III.
[0064] Materials I, II, and III are mixed, and 312.5 grams of 40% (weight) aluminum sol materials are added under stirring, and then 0.1 gram of KOH and 0.06 gram of RuCl are added. 3 50g of the solution, adjust the pH value of the above slurry with ammonia water to make the pH=6.0 of the mixed slurry, after fully stirring, the prepared slurry is carried out into microspheres in a spray dryer according to the usual method, and finally the inner diameter is 89 mm, length is 1700 mm (φ89 * 1700 mm) rotary roaster at 500 ℃ roasting 2.0 hours, the catalyst composition of making is:
[0065] 50 wt% Fe 100 mn 0.1 Zn 5.0 K 0.1 La 0...
Embodiment 3
[0067] Take 587.6 grams of ferric nitrate, add 1000 g of water to dissolve, obtain material I, take 0.4 g of lanthanum nitrate and add 10 g of water to dissolve, obtain material II, take 25.5 grams of 50% manganese nitrate and 21.63 grams of zinc nitrate in the same container, add 100 g of water , stirring and dissolving to obtain material III.
[0068] Materials I, II, and III were mixed, and 312.5 grams of 40% by weight aluminum sol material was added under stirring, and then 0.1 gram of KOH, 0.04 gram of PdCl and 0.06 gram of RuCl were added. 3 50g of the solution, adjust the pH value of the above-mentioned slurry with ammonia water so that the pH=6.0 of the mixed slurry, obtain the slurry after fully stirring, carry out the microsphere molding of the prepared slurry in a spray drier according to the usual method, Inner diameter is 89 millimeters at last, and length is 2.0 hours in 500 ℃ of roastings in the rotary roaster of 1700 millimeters (φ 89 * 1700 millimeters), and t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com