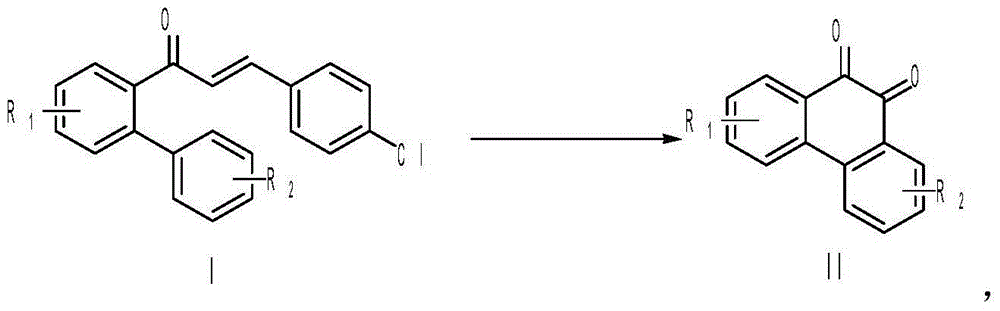
Preparing method of 9,10-phenanthraquinone compound
The technology of a compound, phenanthrenequinone, applied in the field of organic compound synthesis, can solve the problems of many by-products, high cost, and difficulty in obtaining raw materials, and achieve the effects of mild reaction conditions, energy saving, and strong substrate universality
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024]
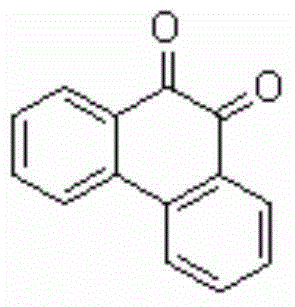
[0025] Add 0.2mmol of 1-p-chlorophenyl-3-(2'-phenyl)phenyl-2-allyl ketone, 0.4mmol of Selectfluor, and 0.02mmol of Cu powder into a 10mL reaction tube, then add 2mL of acetonitrile / water (V:V= 50:1) as solvent. Then, magnetically stir at 25° C. for 12 h. Then, two spoonfuls of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and the pure product was obtained by column chromatography separation (with petroleum ether / ethyl acetate=6:1 as an eluent). The material was an orange-red solid in 76% yield.
[0026] Characterization data: 1 HNMR (CDCl 3 , 400MHz): 8.17-8.15(m, 2H), 7.99(d, J=8Hz, 2H), 7.73-7.68(m, 2H), 7.48-7.44(m, 2H); 13 CNMR (CDCl 3 , 100MHz): δ180.3, 136.0, 135.8, 131.0, 130.5, 129.6, 124.0; MS (EI, 70eV): m / z (%) = 208 [M + ].
Embodiment 2
[0028]
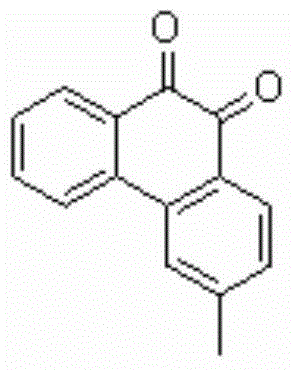
[0029] Add 0.2mmol of 1-p-chlorophenyl-3-(2'-m-methylphenyl)phenyl-2-allyl ketone, 0.4mmol of Selectfluor, and 0.02mmol of Cu powder into a 10mL reaction tube, then add 2mL of acetonitrile / water (V : V=50:1) as solvent. Then, magnetically stir at 25° C. for 12 h. Then, two spoonfuls of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and the pure product was obtained by column chromatography separation (with petroleum ether / ethyl acetate=6:1 as an eluent). The material was an orange-red solid in 80% yield.
[0030] Characterization data: 1 HNMR (CDCl 3 , 400MHz): 8.05-8.03(m, 1H), 7.93(d, J=8Hz, 1H), 7.86(d, J=8Hz, 1H), 7.65(s, 1H), 7.61-7.57(m, 1H) , 7.37-7.33(m, 1H), 7.14(d, J=7.6Hz, 1H), 2.40(s, 3H); 13 CNMR (CDCl 3 , 100MHz): δ179.4, 179.2, 147.6, 142.8, 137.4, 134.4, 131.9, 131.2, 130.9, 129.6, 129.4, 129.1, 124.6, 124.1, 22.4; MS (EI, 70eV): m / z (%) ...
Embodiment 3
[0032]
[0033] Add 0.2mmol of 1-p-chlorophenyl-3-(2'-m-methylphenyl)phenyl-2-allyl ketone, 0.2mmol of Selectfluor, and 0.008mmol of Cu powder into a 10mL reaction tube, then add 2mL of acetonitrile / water (V : V=50:1) as solvent. Then, magnetically stir at 25° C. for 12 h. Then, two spoonfuls of column chromatography silica gel (100-200 mesh) were added to the reaction solution, and the solvent was removed by distillation under reduced pressure, and the pure product was obtained by column chromatography separation (with petroleum ether / ethyl acetate=6:1 as an eluent). The material was an orange-red solid in 56% yield.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com