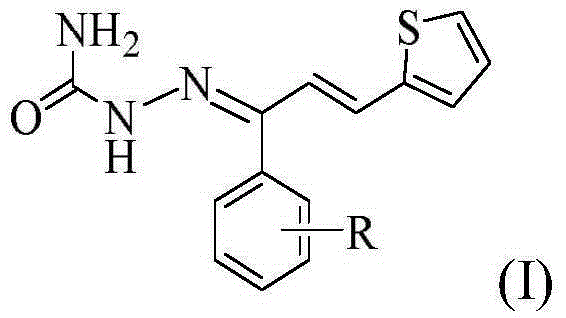
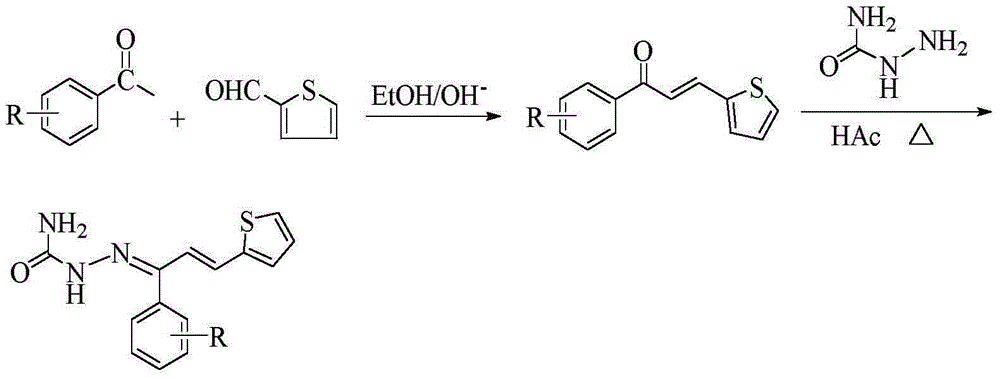
Thiophene chalcone semicarbazone Schiff base compounds, and preparation method and applications thereof
A technology for thiophene chalcone semicarbazide and compound is applied in the field of thiophene chalcone semicarbazide Schiff base compounds and preparation thereof, and achieves the effects of easy synthesis, novel structure and simple structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] compound (C 14 h 12 ON 3 SF) preparation
[0033] (1) Synthesis of intermediate 1-(4-fluorophenyl)-3-(2-thienyl)-2-propen-1-one
[0034] Dissolve 0.02 mol of 4-fluoroacetophenone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. Under ice-bath stirring, the mixture of 0.02mol 2-thiophenecarbaldehyde and 20mL of absolute ethanol was slowly dropped into the above-mentioned mixed solution with a constant pressure dropping funnel, reacted at 0-5°C, and was tested with a thin-layer silica gel plate (TLC ) to check that the reaction is complete. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-fluorophenyl)-3-(2-thienyl)-2-propen-1-one.
[0035] (2) Synthesis of the target compound
[0036] Dissolve 0.015 mol of semicarbazide in 20 mL of 90% ...
Embodiment 2
[0039] compound (C 20 h 17 ON 3 S) Preparation
[0040] (1) Synthesis of intermediate 1-(4-biphenyl)-3-(2-thienyl)-2-propen-1-one
[0041] Dissolve 0.02 mol of biphenyl monoethyl ketone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. Under ice-bath stirring, the mixture of 0.02mol 2-thiophenecarbaldehyde and 20mL of absolute ethanol was slowly dropped into the above-mentioned mixed solution with a constant pressure dropping funnel, reacted at 0-5°C, and was tested with a thin-layer silica gel plate (TLC ) to check that the reaction is complete. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(4-biphenyl)-3-(2-thienyl)-2-propen-1-one.
[0042] (2) Synthesis of the target compound
[0043] Dissolve 0.015 mol of semicarbazide in 20 mL of 90% etha...
Embodiment 3
[0046] compound (C 14 h 12 ON 3 Preparation of SCl)
[0047] (1) Synthesis of intermediate 1-(3-chlorophenyl)-3-(2-thienyl)-2-propen-1-one
[0048] Dissolve 0.02 mol of 3-chloroacetophenone in 30 mL of absolute ethanol, and then add 10 mL of 10% NaOH solution thereto. Under ice-bath stirring, the mixture of 0.02mol 2-thiophenecarbaldehyde and 20mL of absolute ethanol was slowly dropped into the above-mentioned mixed solution with a constant pressure dropping funnel, reacted at 0-5°C, and was tested with a thin-layer silica gel plate (TLC ) to check that the reaction is complete. After the reaction is completed, add 3-4 times the volume of distilled water to the mixture, and adjust its pH value to neutral with 10% HCl, there is a precipitate, filter, wash, and recrystallize with absolute ethanol to obtain intermediate 1 -(3-Chlorophenyl)-3-(2-thienyl)-2-propen-1-one.
[0049] (2) Synthesis of the target compound
[0050] Dissolve 0.015 mol of semicarbazide in 20 mL of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com