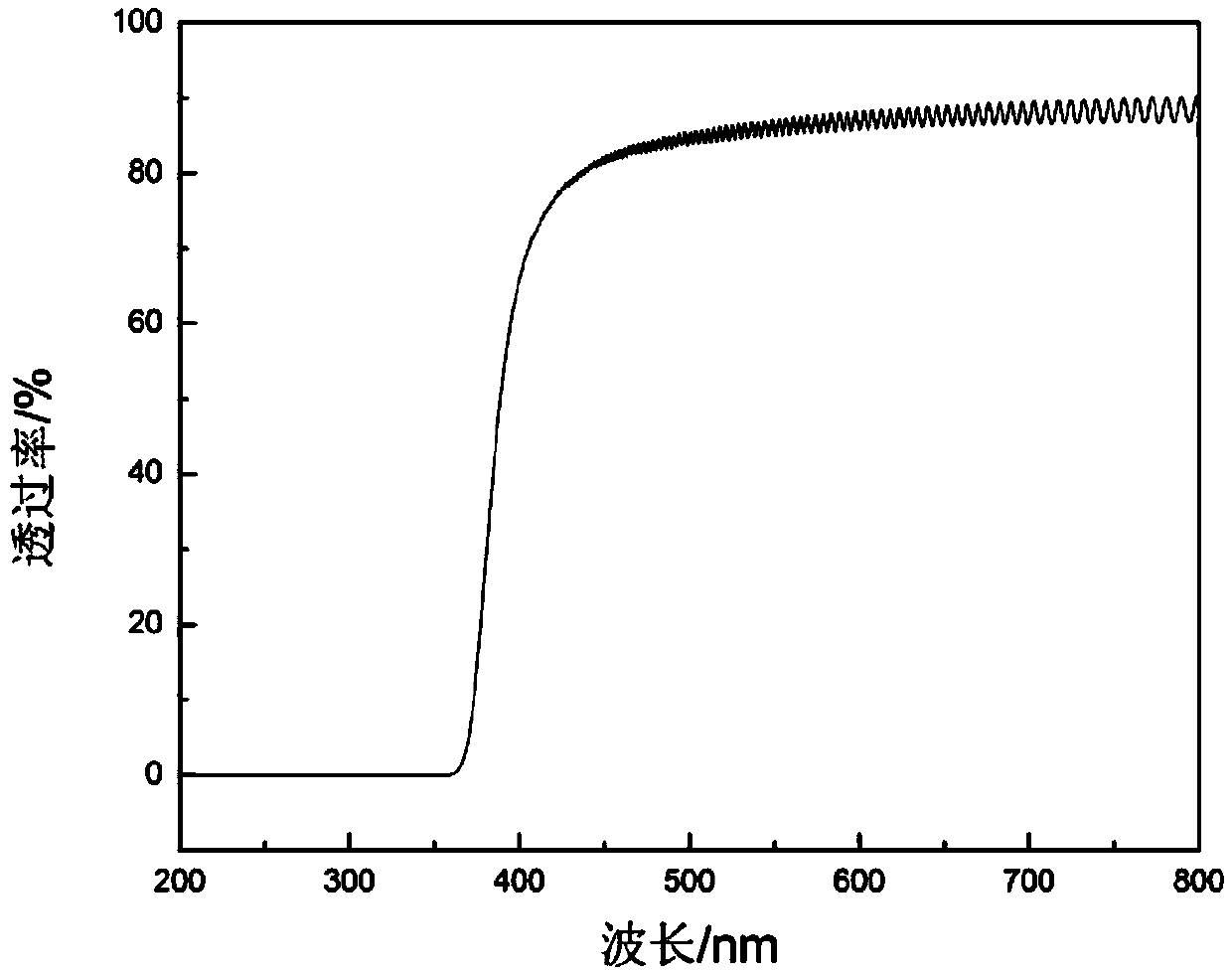
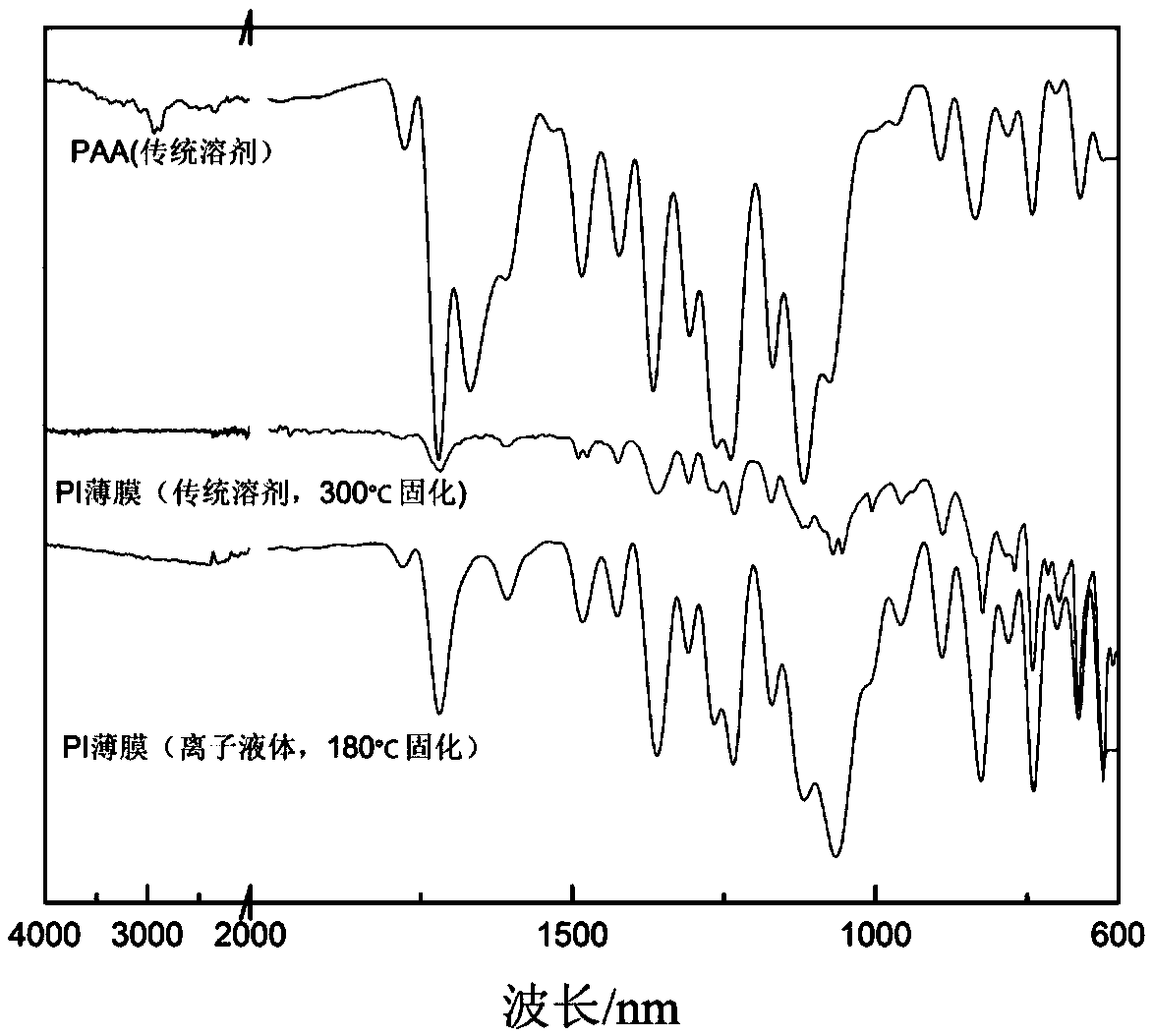
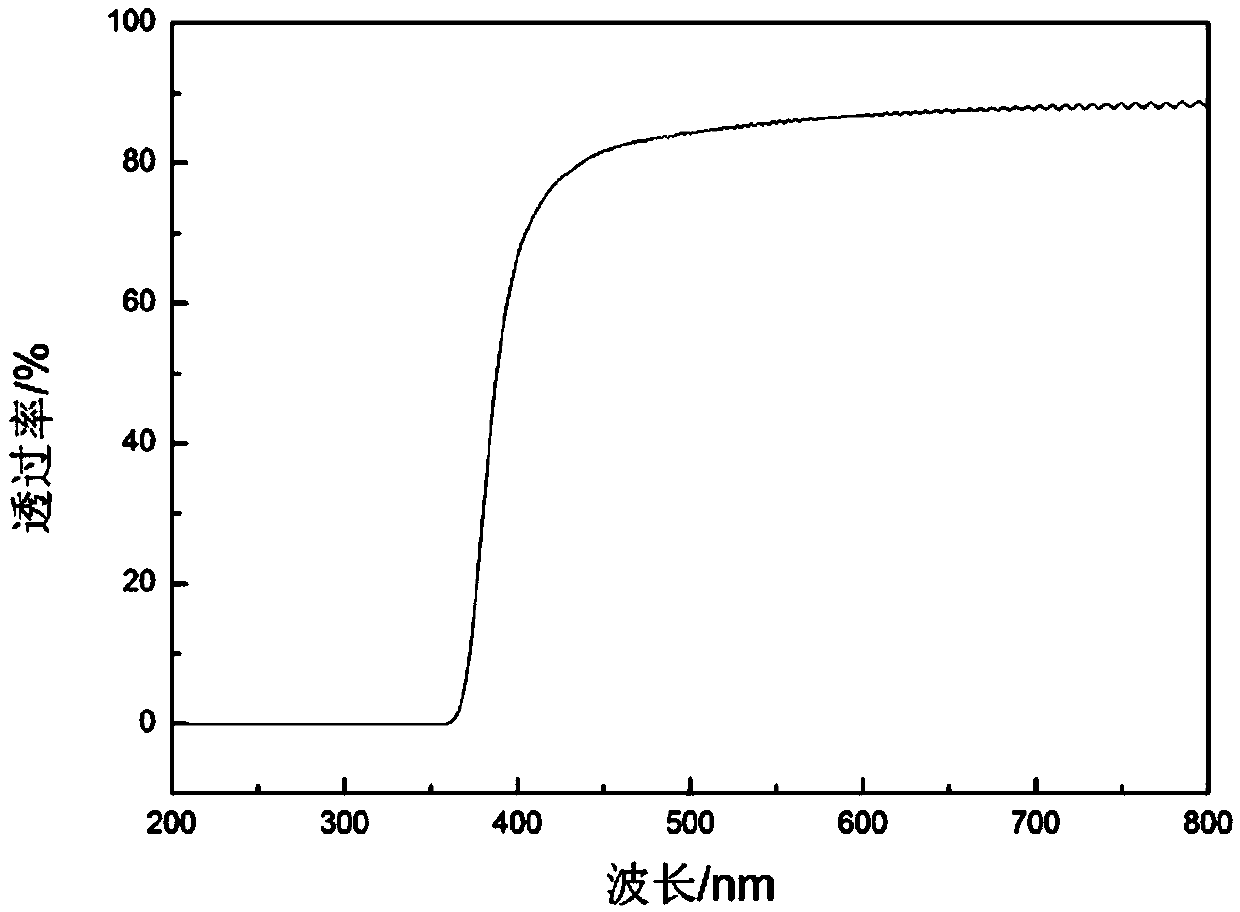
Polyimide synthetic method
A technology of polyimide and synthesis method, which is applied in the field of polyimide synthesis, can solve the problems of poor transparency of polyimide, and achieve the effect of improving transparency and high transparency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Introduce nitrogen into a 250mL three-neck flask equipped with a mechanical stirring device, first add 2.9112g (0.0091mol) 2,2'-bis(trifluoromethyl)diaminobiphenyl and 0.2422g (0.0009mol) 1,1' - Mixed diamines of bis(4-aminophenyl)cyclohexane, followed by 62.56 g of ionic liquid 1,3-bis(2-methoxy-2-oxoethyl)imidazole tetrafluoroborate, 1 , The structural formula of 3-bis(2-methoxy-2-oxyethyl)imidazolium tetrafluoroborate is:
[0035]
[0036] Turn on the mechanical stirring and heat to make it dissolve. At this time, the temperature of the mixed solution in the three-neck flask is not higher than 50°C. After the mixed diamine is completely dissolved, add 3.1021g (0.01mol) 3,3',4,4'-diphenyl ether tetracarboxylic dianhydride into the three-necked flask, stir for 10min, squeeze the nitrogen bag to make the three-necked flask Remove as much air as possible. The mixture in the three-necked flask was slowly heated up to 180° C., stirred at a constant temperature of 180°...
Embodiment 2
[0041] Argon was passed into a 250mL three-neck flask equipped with a mechanical stirring device, and 1.6012g (0.005mol) of 2,2'-bis(trifluoromethyl)diaminobiphenyl and 1.3319g (0.005mol) of 1,1 The mixed diamine of '-bis(4-aminophenyl) cyclohexane, then add 14.75g ionic liquid 1,3-bis(2-methoxy-2-oxoethyl) imidazole tetrafluoroborate, And turn on the mechanical stirring, heat to make it dissolve, and the temperature of the mixed solution in the three-necked flask is not higher than 50°C at this time. After the mixed diamine is completely dissolved, add 4.4424g (0.01mol) 4,4'-hexafluoroisopropyl phthalic anhydride to the three-necked flask, and after stirring for 10min, squeeze the argon bag to make the air in the three-necked flask Eliminate as much as possible. The mixed solution in the three-neck flask was slowly heated up to 150° C., stirred at a constant temperature of 150° C. for 10 h, and then cooled down to room temperature naturally to obtain a pale yellow polyimide ...
Embodiment 3
[0045] Nitrogen was passed into a 250mL three-neck flask equipped with a mechanical stirring device, and 2.6579g (0.0083mol) of 2,2'-bis(trifluoromethyl)diaminobiphenyl and 0.4528g (0.0017mol) of 1,1' - Mixed diamines of bis(4-aminophenyl)cyclohexane, and then add 17.21g of ionic liquid chloride 1,3-bis(2-methoxy-2-oxyethyl)imidazole chloride, chloride 1, The structural formula of 3-bis(2-methoxyl-2-oxyethyl)imidazole is:
[0046]
[0047] Turn on the mechanical stirring and heat to make it dissolve. At this time, the temperature of the mixed solution in the three-neck flask is not higher than 50°C. After the mixed diamine is completely dissolved, add 1.5511g (0.005mol) of 3,3',4,4'-diphenyl ether tetracarboxylic dianhydride and 2.2212g (0.005mol) of 4,4'-hexafluoroiso Propyl phthalic anhydride, after stirring for 10 minutes, squeeze the nitrogen bag to remove the air in the system as much as possible. The mixed solution in the three-necked flask was slowly heated up to 2...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
| transmittivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com