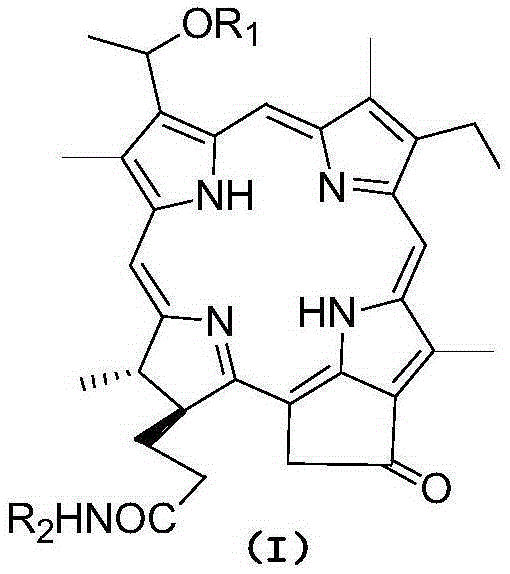
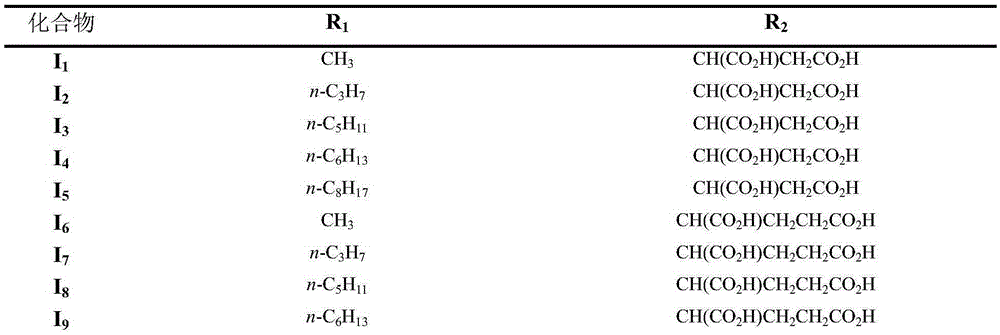
Pyropheophorbide-a ether amino acid derivative as well as preparation method and application thereof
A technology of pyropheophorbide and amino acid, which is applied in the field of medicine, can solve the problems of short tumor killing depth, low photosensitivity activity, high retention phototoxicity, etc., and achieves the effect of excellent photodynamic killing effect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Embodiment 1: Preparation of pyropheophorbide a (V)
[0057] Silkworm excrement paste chlorophyll was purchased from Zhejiang Haining Fengming Chlorophyll Co., Ltd.
[0058] Method 1: Add 100g of silkworm excrement paste chlorophyll, add 500mL of ether and an equal volume of concentrated hydrochloric acid, pass N 2 After 2 days of airtight reaction, the reaction solution was poured into a separatory funnel and left to stand to separate the lower layer of acid water, diluted with 2 times the amount of water, neutralized with 10mol / L NaOH to a pH of 5-6 under cooling, suction filtered, P 2 o 5 After drying, it was separated by silica gel H column chromatography [the mobile phase was CH 2 Cl 2 :CH 3 COCH 3 :CH 3 OH: HCO 2 H=60:1:1:0.1 (v / v / v / v)] to obtain 2 g of pure compound V.
[0059] MS (ESI + ) m / z: 535.54 (M+H, 100%); 1068.98 (2M+1, 100%).
[0060] 1 HNMR (300MHz, CDCl 3 ,δ,ppm):9.36(1H,s,meso-H),9.26(1H,s,meso-H),8.50(1H,s,meso-H),7.91(1H,dd,J=18.0,9.0 ...
Embodiment 2
[0062] Example 2: 3 1 -Bromoethyl-3 1 - Preparation of devinylpyropheophorbide a(IV)
[0063] Compound V (1.0 g) was added with 100 mL of 33% HBr glacial acetic acid solution, sealed at room temperature for 24 hours, and the glacial acetic acid was evaporated under reduced pressure to obtain dark green solid compound IV, which was directly used in the next reaction without purification.
Embodiment 3
[0064] Example 3: 3 1 -Methoxy-3 1 - Devinylpyropheophorbide a(Ⅲ 1 ) preparation
[0065] The above dark green solid compound IV (0.5g), dissolved in 50mL of dry DCM, added 1gK 2 CO 3 and 10 mL of dry methanol in N 2 The reaction was carried out at room temperature under protection, and the progress of the reaction was monitored by TLC. After about 2 hours, the reaction was completed and the reaction was stopped. Add 5 times the volume of water to the reaction solution, extract with DCM (200mL×3), separate and combine the organic layers, wash 3 times with water, wash 1 time with saturated brine, anhydrous Na 2 SO 4 After drying for 2h, the organic solvent was recovered under reduced pressure and separated by silica gel H chromatography, mobile phase DCM:CH 3 COCH 3 :CH 3 OH:CH 3 CO 2 H=80:1:1:0.1(v / v / v / v), get 0.3g black powderⅢ 1 , melting point 230-231°C, yield 56.6%.
[0066] MS (ESI + ) m / z: 567.66 (M+H, 100%), 1133.93 (2M+1, 35%).
[0067] 1 HNMR (300MHz, ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com