A kind of chlorin e6 derivative and its pharmaceutically acceptable salt, its preparation method and application
A technology of chlorin and its derivatives, which is applied in the field of medicine, can solve the problems of reduced toxicity, high residual phototoxicity, short laser penetration and killing tumor depth, etc., and achieves reduced toxicity, improved therapeutic effect, and excellent photodynamic killing effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
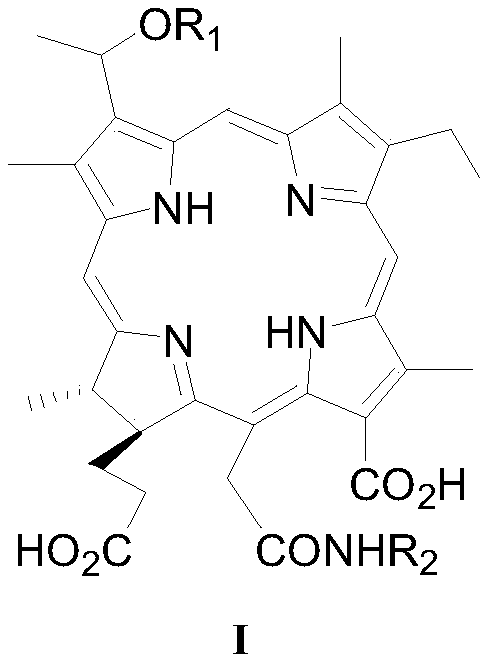
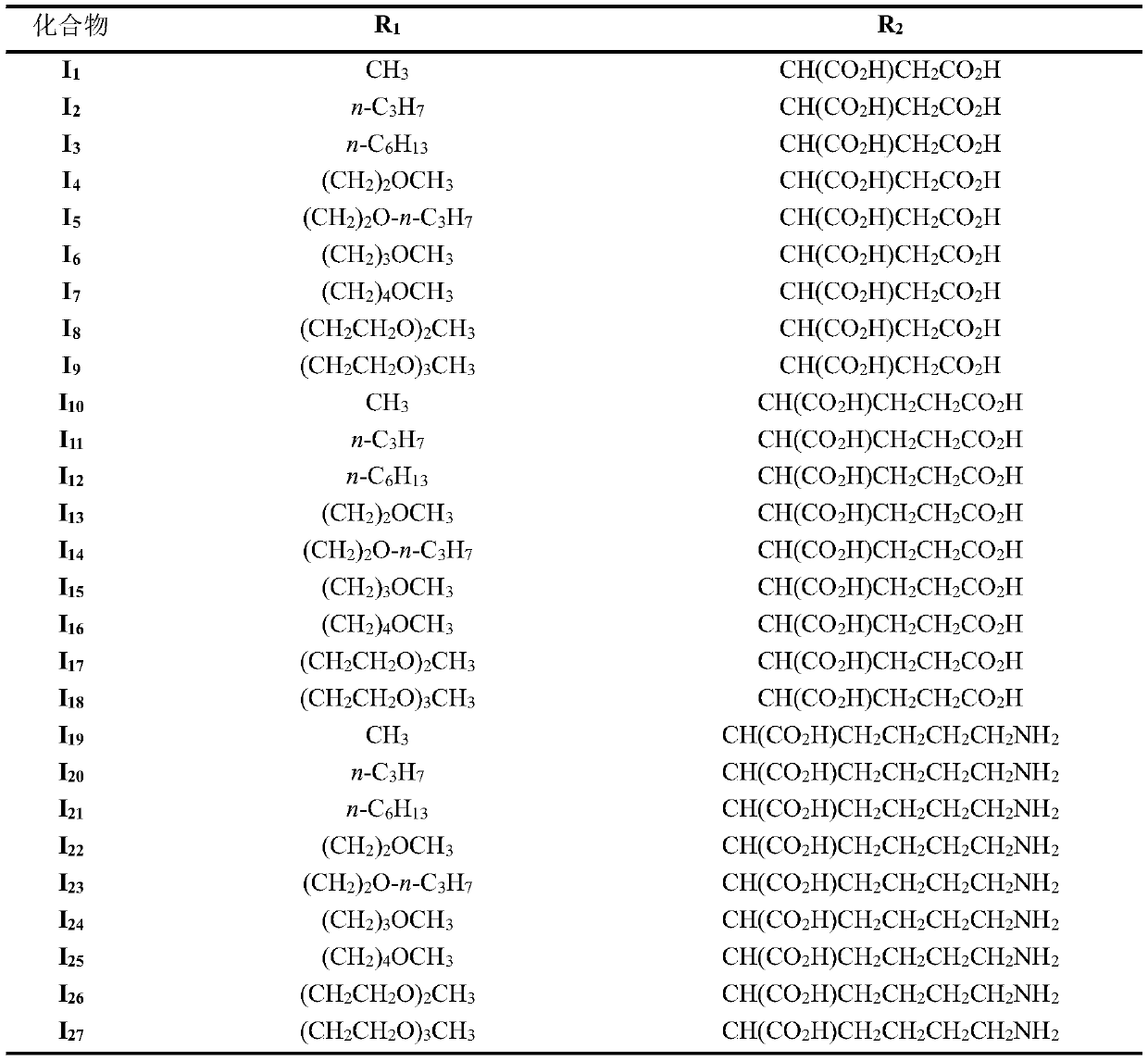
[0052] Example 1: N-[3-(1-methoxy)ethyl-3-desvinyl chlorin e6-15 2 -acyl]-L-aspartic acid (I 1 ) preparation
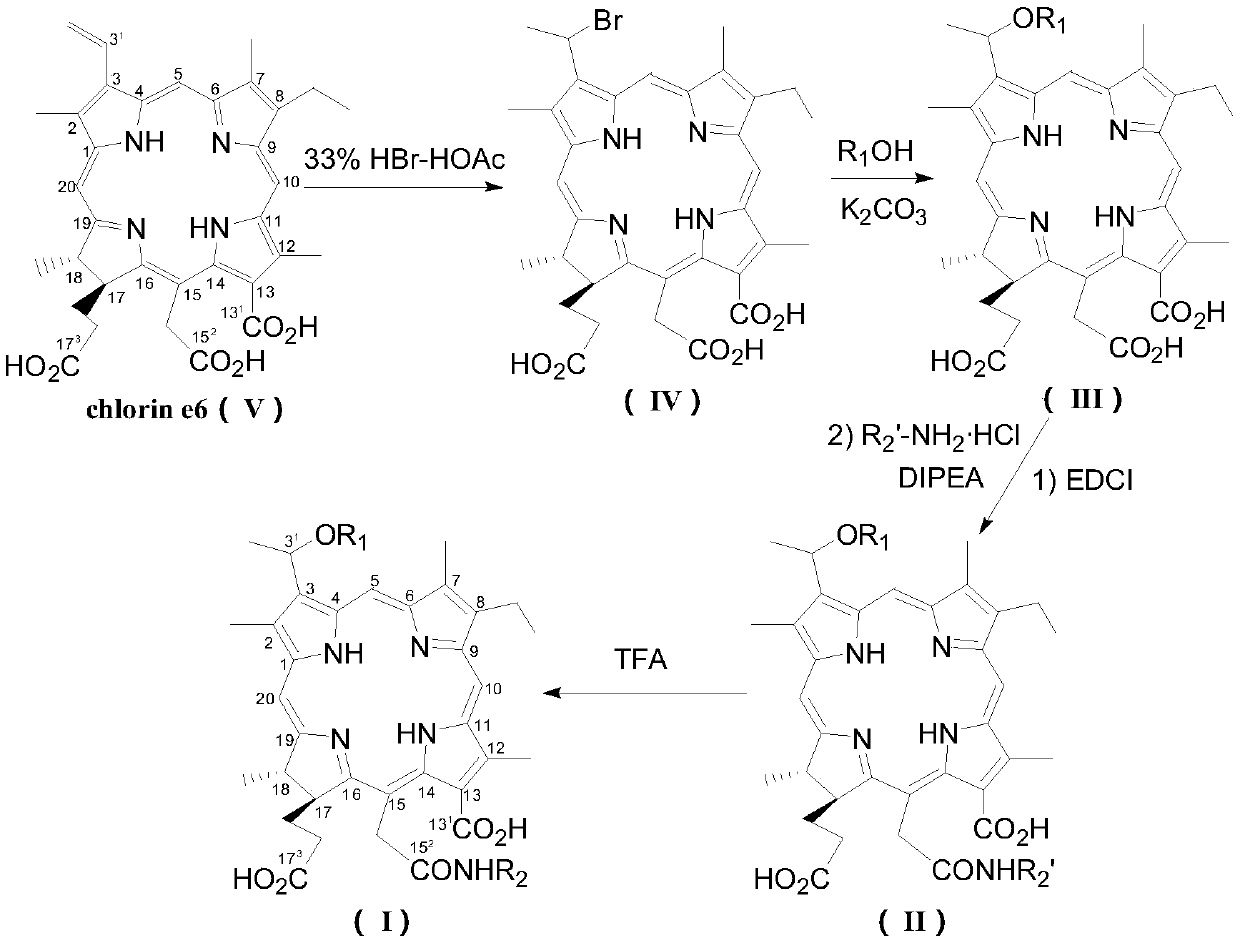
[0053] S1: Preparation of 3-(1-bromoethyl)-3-desvinylchlorin e6(IV)
[0054] Compound Ⅴ (5.0g), add 200mL of 33% HBr glacial acetic acid solution, seal and stir at room temperature for 24h, evaporate glacial acetic acid and excess HBr under reduced pressure to obtain 5.6g dark green solid compound Ⅳ, namely 3-(1-bromoethyl base)-3-desvinyl chlorin e6, which was directly used in the next reaction without further purification.
[0055] S2: 3-(1-methoxy)ethyl-3-desvinylchlorin e6(Ⅲ 1 ) preparation
[0056] Compound IV (1.12g), dissolved in 25mL of anhydrous acetone, added 2g K 2 CO 3 and 2 mL of dry methanol, stirred and refluxed for 2 h, cooled to room temperature, added 10 times the volume of water, and washed with 10% H 2 SO 4 Neutralize excess K 2 CO 3 And adjust the pH to 5-6, filter, P 2 o 5 After vacuum drying, it was separated by silica gel H column c...
Embodiment 2
[0064] Example 2: N-[3-(1-n-propoxy)ethyl-3-desvinyl chlorin e6-15 2 -acyl]-L-aspartic acid (I 2 ) preparation
[0065] S1: The preparation of 3-(1-bromoethyl)-3-desvinyl chlorin e6(IV) is the same as the preparation method of step S1 in Example 1;
[0066] S2: 3-(1-propoxy)ethyl-3-desvinylchlorin e6(Ⅲ 2 ) preparation: according to the method of step S2 of Example 1, compound IV (1.12g) was reacted with 2mL dry n-propanol to obtain 0.56g black solid III 2 , yield 50.9%.
[0067] 3-(1-propoxy)ethyl-3-desvinyl chlorin e6(Ⅲ 2 ) spectrum data is: MS(ESI + ) m / z:657.78[M+H] + (100%).
[0068] S3: N-[3-(1-n-propoxy)ethyl-3-desvinylchlorin e6-15 2 -Acyl]-L-aspartic acid di-tert-butyl ester (Ⅱ 2 ) preparation
[0069] According to the method of embodiment 1 step S3, compound III 2 (140mg, 0.213mmol) was reacted with equivalent EDCI, 1.2 equivalents of L-aspartic acid di-tert-butyl hydrochloride and 2.4 equivalents of DIPEA in dry DMF to obtain black powder II 2 125 mg, yie...
Embodiment 3
[0074] Example 3: N-[3-(1-n-hexyloxy)ethyl-3-desvinyl chlorin e6-15 2 -acyl]-L-aspartic acid (I 3 ) preparation
[0075] S1: The preparation of 3-(1-bromoethyl)-3-desvinyl chlorin e6(IV) is the same as the preparation method of step S1 in Example 1;
[0076] S2: 3-(1-n-hexyloxy)ethyl-3-desvinylchlorin e6(Ⅲ 3 ) preparation: according to the method of step S2 of Example 1, compound IV (1.12g) was reacted with 2mL dry n-hexanol to obtain 0.48g black solid III 3 , yield 41.0%.
[0077] Compound III 3 The spectrogram data is: MS(ESI + )m / z:699.68[M+H] + (100%).
[0078] S3: N-[3-(1-n-hexyloxy)ethyl-3-desvinylchlorin e6-15 2 -Acyl]-L-aspartic acid di-tert-butyl ester (Ⅱ 3 ) preparation: according to the method of embodiment 1 step S3, compound III 3 (150mg, 0.215 mmol) was reacted with equivalent EDCI, 1.2 equivalents of L-aspartic acid di-tert-butyl hydrochloride and 2.4 equivalents of DIPEA in dry DMF to obtain black powder Ⅱ 3 120 mg, yield 60.4%.
[0079] Compound II...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com