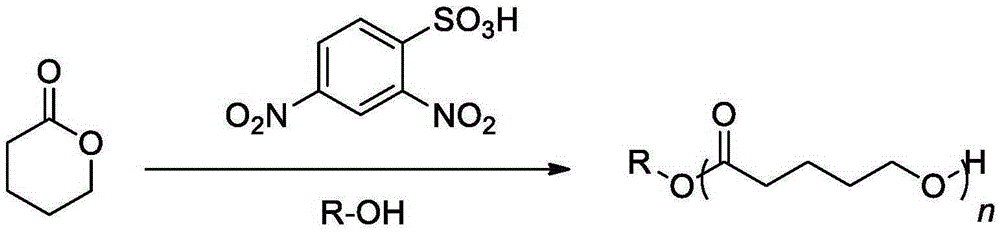
Method for preparing polylactone through ring-opening polymerization
A technology of ring-opening polymerization and polylactone is applied in the field of organic sulfonic acid catalyzing lactone to prepare polylactone, and achieves the effects of low dispersion, mild reaction conditions and high conversion rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
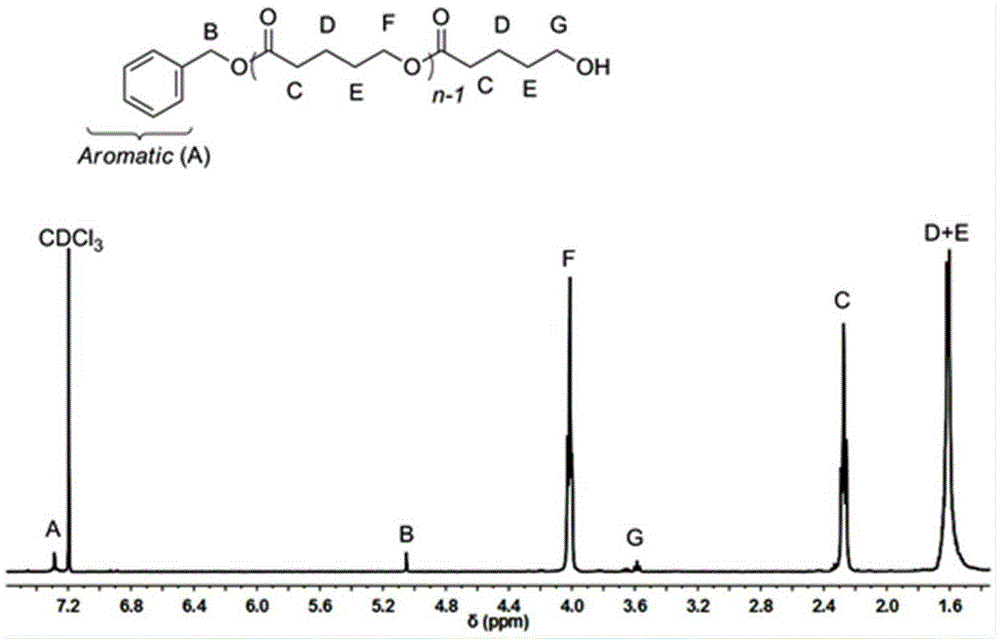
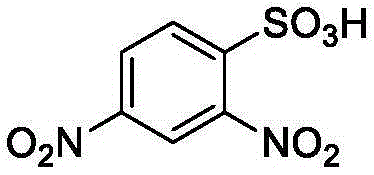
[0023] In a 10mL single-necked bottle, add δ-valerolactone (0.181ml, 2mmol), acetonitrile 2mL, 2.4-dinitrobenzenesulfonic acid (0.0124g, 0.05mmol), benzyl alcohol (5.2ul, 0.05mmol), monomer The concentration was 1 mol / L, and mechanically stirred at 25°C for 8 hours. After the reaction was over, the reaction was terminated. The reaction solution was rotary evaporated, and the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifuge to obtain a white solid, which is transferred to a vacuum oven for drying. The conversion rate was 53.1%. The final polymer obtained by 1 HNMR identification, molecular weight and dispersion properties of the polymer were determined by size exclusion chromatography.
Embodiment 2
[0025] In a 10mL single-necked bottle, add δ-valerolactone (0.181ml, 2mmol), acetonitrile 1mL, 2.4-dinitrobenzenesulfonic acid (0.0124g, 0.05mmol), benzyl alcohol (5.2ul, 0.05mmol), monomer The concentration was 2 mol / L, and mechanically stirred for 10 hours at 25°C. After the reaction was over, the reaction was terminated. The reaction solution was rotary evaporated, and the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifuge to obtain a white solid, which is transferred to a vacuum oven for drying. The conversion rate was 72.9%. The final polymer obtained by 1 HNMR identification, molecular weight and dispersion properties of the polymer were determined by size exclusion chromatography.
Embodiment 3
[0027] In a 10mL single-necked bottle, add δ-valerolactone (0.181ml, 2mmol), acetonitrile 0.67mL, 2.4-dinitrobenzenesulfonic acid (0.0124g, 0.05mmol), benzyl alcohol (5.2ul, 0.05mmol), single The volume concentration was 3 mol / L, and mechanically stirred for 10 hours at 25°C. After the reaction was over, the reaction was terminated. The reaction solution was rotary evaporated, and the obtained crude product was dissolved in a minimum amount of dichloromethane, and then added into a cold methanol solution, and a polymer was precipitated. Centrifuge to obtain a white solid, which is transferred to a vacuum oven for drying. The conversion rate was 85.3%. The final polymer obtained by 1 HNMR identification, molecular weight and dispersion properties of the polymer were determined by size exclusion chromatography.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com