Coated particles and compositions comprising same
A particle and polymer technology, applied in the directions of heterocyclic compound active ingredients, peroxy compound active ingredients, drug delivery, etc., can solve problems such as high recurrence rate and high application frequency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0277] CLAIMS 1. A particle comprising: (i) a core comprising a first active agent; and (ii) a first lipid-containing coating at least partially covering the core.
[0278] 2. A particle comprising: (i) a core comprising a first active agent; and (ii) a first carbohydrate-containing coating at least partially covering the core.
[0279] 3. A particle comprising: (i) a core comprising a first active agent; and (ii) a first coating comprising a protein at least partially covering the core.
[0280] 4. A particle comprising: (i) a core comprising a first active agent; and (ii) a first coating comprising cationic molecules at least partially covering the core.
[0281] 5. A particle comprising: (i) a core comprising a first active agent; and (ii) a first coating comprising at least two molecules selected from the group consisting of lipids, carbohydrates, proteins and cationic molecules and the coating The nucleus is at least partially covered.
[0282] 6. The particle of any of...
Embodiment 1
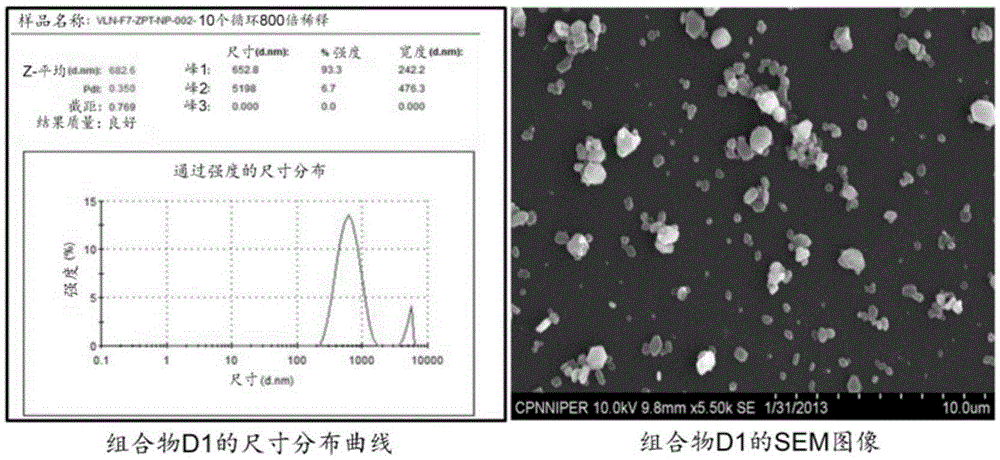
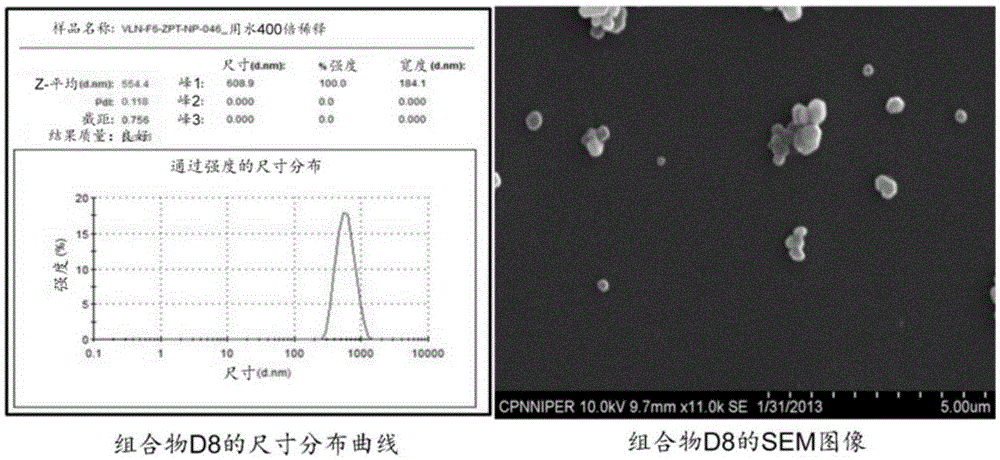
[0517] Example 1: Lipid-Coated Zinc Pyrithione (ZPT) Particle Dispersions (Compositions D1-D11)
[0518] Preparation: Lipids and mixtures with 1% docusate sodium (and 2% ovalbumin, if desired) in water were heated under continuous vigorous stirring to melt the lipids. Add the ZPT powder in portions to the stirring hot mixture. The resulting suspension is passed through a high pressure homogenizer at about 1200-1500 bar. The output dispersion was collected in a beaker kept in an ice bath and recirculated about 6-10 times to produce a dispersion of particles of the appropriate size (about 200 nm to about 800 nm). Size distribution was determined by ZetaSizer (ZS-90 from Malvern Instruments) and scanning electron microscope (SEM, Hitachi, S-3400N, Japan), as figure 1 and 2 shown in . The resulting dispersion was stabilized by the addition of a carbomer solution followed by neutralization with sodium hydroxide to a pH ranging from about 6.5 to about 7.0. In Table 1 some data ...
Embodiment 2
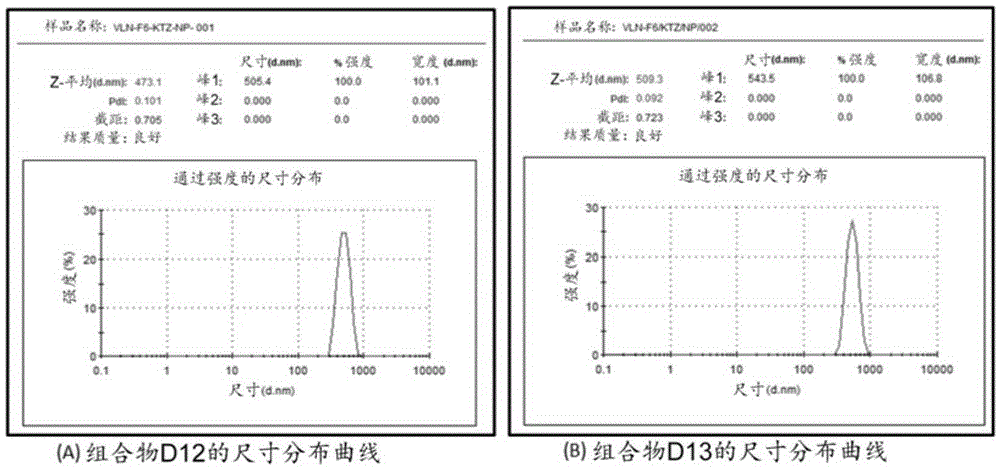
[0522] Example 2: Lipid-Coated Ketoconazole (KTZ) Particle Dispersions (Compositions D12-D16)
[0523] Preparation: A mixture of lipid and 1% aqueous solution of docusate sodium (or polyvinyl alcohol) was heated under continuous vigorous stirring to melt the lipid. Add the KTZ powder in portions to the stirring hot mixture. The resulting suspension is passed through a high pressure homogenizer at about 1200-1500 bar. The output dispersion was collected in a beaker kept in an ice bath and recirculated about 6-10 times to produce a dispersion of particles of the appropriate size (about 300 nm to about 700 nm). Determine the size distribution by ZetaSizer (ZS-90 from Malvern Instruments), as image 3 A and 3B are shown. The resulting dispersion was stabilized by the addition of a carbomer solution followed by neutralization with sodium hydroxide to a pH ranging from about 6.5 to about 7.0. In Table 2 some data for this example are given.
[0524] Table 2: Dispersion composit...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com