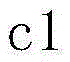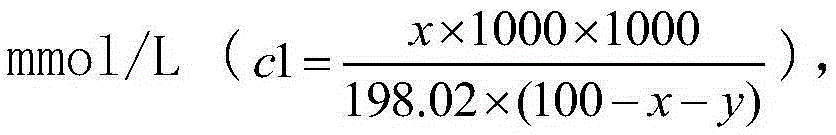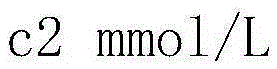Physiological hydrated alginate hydrogel for assistant heart failure treatment and preparation method thereof
A technology of alginate and adjuvant therapy, applied in the field of physiologically hydrated alginate hydrogel and its preparation, can solve the problems of high price, unstoppable left ventricular function continuous attenuation, high surgical risk, etc. effect of change
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] In this embodiment, when the concentration of sodium alginate solution is less than 3.1% w / w (x c 1 m m o l / L ( c 1 = x × 1000 × 1000 198.02 × ( 100 - x - y ) ) , The concentration of sodium ions contributed by sodium chloride is out c 2 m m o l / ...
Embodiment 2
[0031] In this example, when preparing the calcium alginate system, no sodium chloride was added, that is, the sodium ion concentration in the calcium alginate system was 0 mmol / L, and the sodium alginate system was responsible for the source of all sodium ions in the solution in the crosslinked colloid. Assuming that the volume required for the final sodium alginate system is v1 and the calcium alginate system is v2, it is required that the sodium ion concentration of the final system needs to reach cmmol / L (140 The sodium ion concentration provided by sodium chloride in the sodium alginate system is After the sodium alginate system and calcium alginate are mixed, the total number of moles of sodium ions is nmol (n=v1×(c1+c2)), and the concentration of sodium ions after mixing should be Based on this, the amount of sodium alginate and sodium chloride required for one liter of water for injection can be calculated.
[0032] The preparation method of the physiologically hydra...
Embodiment 3
[0038] The preparation method of the injectable alginate-based biomaterial for heart failure treatment of the present invention is as follows.
[0039] In the first step, the concentration of sodium alginate in the sodium alginate system is set to 4% w / w, the concentration of the calcium alginate suspension is 2% w / w, and the volume ratio of the sodium alginate system to the calcium alginate system is 8:8 , the final sodium ion concentration is 150mmol / L. The concentration of the sodium alginate system has exceeded the concentration in Solution 1, so the solution 2 is adopted. Sodium chloride is not added to the calcium alginate system, and all sodium sources are provided by the sodium alginate system. Firstly, the sodium ion concentration provided by sodium alginate in the sodium alginate system is ( c 1 = 4 × 1000 × 1000 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com