Neuroprotective multifunctional antioxidants and their monofunctional analogs
一种抗氧化剂、神经保护的技术,应用在抗毒剂、神经系统疾病、神经肌肉系统疾病等方向,能够解决氧化损害等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0066] N-Benzyl-4,6-dimethoxypyrimidin-2-amine
[0067] (compound 28)
[0068] 2-Chloro-4,6-dimethoxypyrimidine (27) (50.0g, 0.29mol), BnNH 2 (93.3ml, 0.85mol) and potassium carbonate (2.5g, 0.45mol) in dioxane (1.0L) was refluxed for 4 days. The reaction was filtered and the filtrate was concentrated in vacuo to give a yellow oil, which was then purified by silica gel column chromatography with 20:1 to 10:1 hexane:EtOAc to yield 61.7 g (87%) of 28 as a white solid. 1 HNMR (CDCL 3 ) δ 7.36-7.27 (m, 5H), 5.42 (s, 1H), 5.25 (s, 1H), 4.61 (d, J=5.86Hz, 2H), 3.83 (s, 6H).
Embodiment 2
[0070] 2-amino-4,6-dimethoxypyrimidine
[0071] (compound 22)
[0072] Compound 28 (27.0 g, 0.11 mol) in 400 ml MeOH was dissolved in 5.4 g 20% Pd(OH 2 ) hydrogenation at room temperature for 2 days in the presence of a catalyst. After filtration and solvent evaporation, after silica gel column chromatography by using compound 22 in 50:1 CHCl 3 : 16.8 g*98% from MeOH) to obtain a white solid. 1 HNMR (CDCL 3 ) δ 5.47 (s, 1H, 4.90 (s, 2H), 3.84 (s.6H).
Embodiment 3
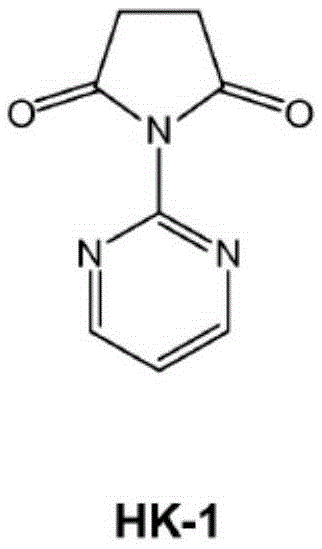
[0074] Synthesis of HK-1, HK-3, HK-5, and HK-7
[0075] The following describes l-(2-pyrimidinyl)pyrrolidine-2,5-dione (HK-1), l-(4,6-dimethoxy-2-pyrimidinyl)pyrrolidine-2,5 -Diketone (HK-3), l-(2-pyrimidinyl)piperidine-2,6-dione (HK-5), l-(4,6-dimethoxy-2-pyrimidinyl)piperidine Pyridine-2,6-dione (HK-7), l-(5-benzyloxy-2-pyrimidinyl)pyrrolidine-2,5 (compound 23), l-(5-benzyloxy-2-pyrimidinyl) piperidine-2,6-dione (compound 24), l-(4,6-dimethoxy-5 benzyloxy-2 pyrimidinyl)pyrrolidine-2,5-dione (compound 25), and l - General synthesis of (4,6-dimethoxy-5benzyloxy-2-pyrimidinyl)piperidine-2,6-dione (compound 26).
[0076] refer to image 3 , 42.0g (0.42mol) of succinic anhydride (17) was dissolved in 300ml of toluene, 200ml of acetone dissolved with 20g (0.21mol) of 2-aminopyrimidine (21) was added, and the mixture was heated to 85°C for 3 days. After cooling to (room temperature) the product precipitated, filtered and washed with toluene. The filtrate was dried under vacuum...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com