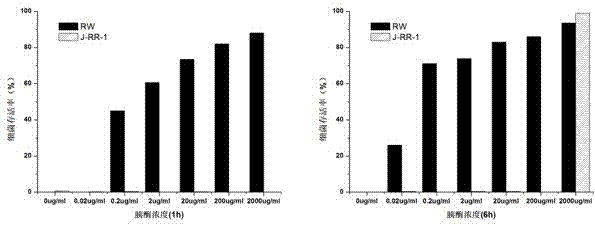
Preparation and application of combined antibacterial peptide having high stability and anti-drug-resistance activity
A high-stability, antimicrobial peptide technology, applied in the field of biochemistry, can solve problems such as limited application, and achieve strong antibacterial activity, high stability, and strong anti-drug-resistant bacterial activity in clinical isolation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Embodiment 1, the synthesis of combination antimicrobial peptide J-RR-1
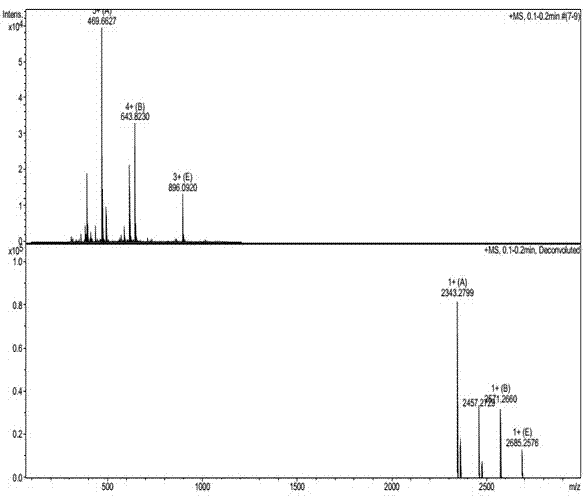
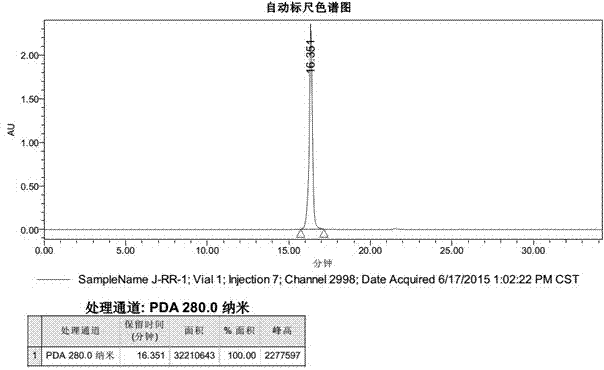
[0039] with H 2 O and DMF (100:1, V / V) are the reaction medium, CuSO 4 ·5H 2 O is catalyst (consumption is peptide Ac-Nle(N 3 )-RW-1 molar weight 10 times), sodium ascorbate is antioxidant (dosage is peptide Ac-Nle(N 3 )-RW-1 molar amount of 15 times), the purified peptide Ac-Pra-RW-1 and peptide Ac-Nle(N 3 )-RW-1 with a molar ratio of 1:1, after 24h of click chemical 1,3-dipolar cycloaddition reaction, the reaction solution was purified by RP-HPLC to obtain the target peptide J-RR-1, and the yield was 18.4%. The purity is 100.00%. The mass spectrum of the product is shown in figure 1 , the purity analysis chart see figure 2 .
Embodiment 2
[0040] Embodiment 2, the synthesis of combination antimicrobial peptide J-RR-1
[0041] with H 2 O and DMF (20:1, V / V) are the reaction medium, CuSO 4 ·5H 2 O is a catalyst (amount is peptide Ac-Nle(N 3 )-RW-1 molar weight 5 times), sodium ascorbate is antioxidant (dosage is peptide Ac-Nle(N 3 )-RW-1 molar amount 20 times), the purified peptide Ac-Pra-RW-1 and peptide Ac-Nle(N 3 )-RW-1 with a molar ratio of 2:1, after 28 hours of click chemical 1,3-dipolar cycloaddition reaction, the reaction solution was purified by RP-HPLC to obtain the target peptide J-RR-1, and the yield was 19.6%, yielding a purity of 100.00%. Spectrum see figure 1 , the purity analysis chart see figure 2 .
Embodiment 3
[0042] Embodiment 3, the synthesis of combination antimicrobial peptide J-RR-1
[0043] with H 2 O and DMF (10:1, V / V) are the reaction medium, CuSO 4 ·5H 2 O is a catalyst (amount is peptide AC-Nle(N 3 )-RW-1 molar weight 15 times), sodium ascorbate is antioxidant (dosage is peptide Ac-Nle(N 3 )-RW-1 molar amount 30 times), the purified peptide Ac-Pra-RW-1 and peptide Ac-Nle(N 3 )-RW-1 with a molar ratio of 5:1, after 26 hours of click chemical 1,3-dipolar cycloaddition reaction, the reaction solution was purified by RP-HPLC to obtain the target peptide J-RR-1, and the yield was 18.2%, the purity is 100.00%. The mass spectrum of the product is shown in figure 1 , the purity analysis chart see figure 2 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com