Human metallothionein-4 fusion protein expression vector
A metallothionein and expression vector technology, applied in the fusion expression of human free fatty acid binding protein and human metallothionein, and the field of human metallothionein-4 fusion protein expression vector, can solve the problems of high cost and low yield.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] A method for constructing a fusion protein expression vector of a chaperone-like protein, comprising the following steps:
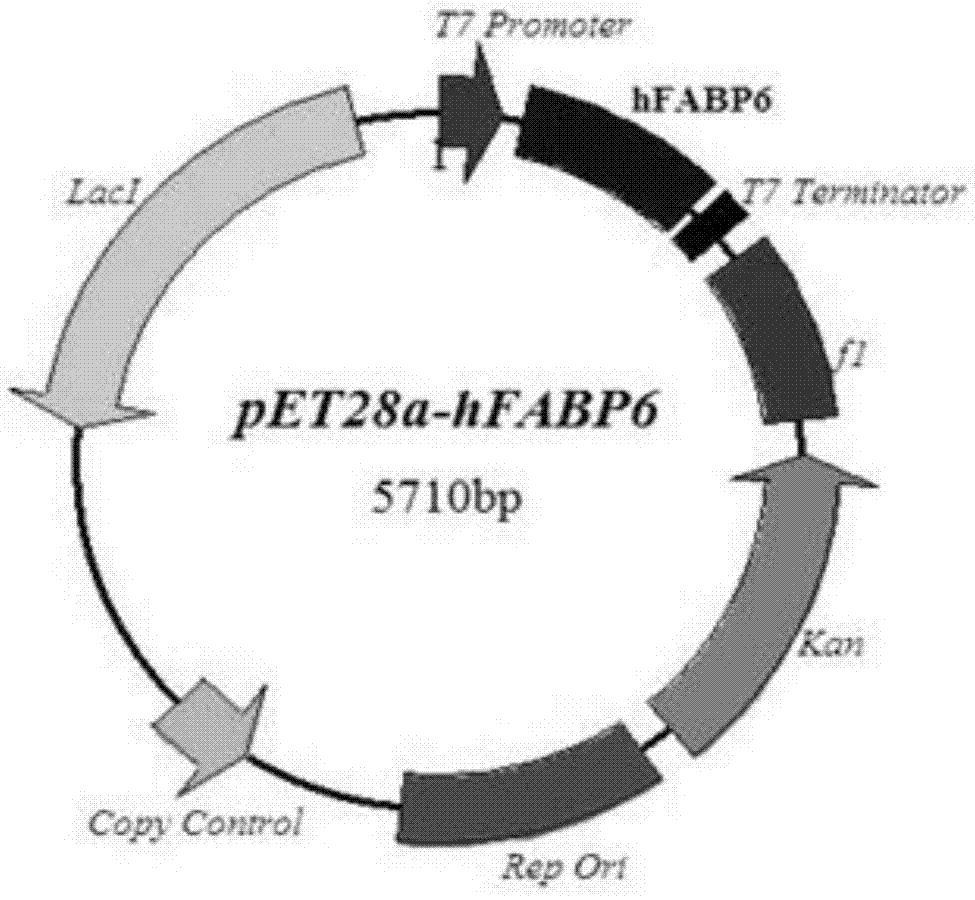
[0027] Step 1. Artificial synthesis and optimization of hFABP-encoding DNA. In this embodiment, hFABP6 is taken as an example:
[0028] (1) Take the hFBP6 amino acid sequence (SEQ ID NO.2) and reverse translate it into a DNA coding sequence, and obtain the sequence (SEQ ID NO.1) after codon priority and ribosome binding region sequence optimization, but not limited to this sequence, and use the translated protein The amino acid sequence homology is equal to or greater than 85%.
[0029] (2) Segment the coding sequence to synthesize oligonucleotide single-stranded DNA (SEQ ID NO.5-18).
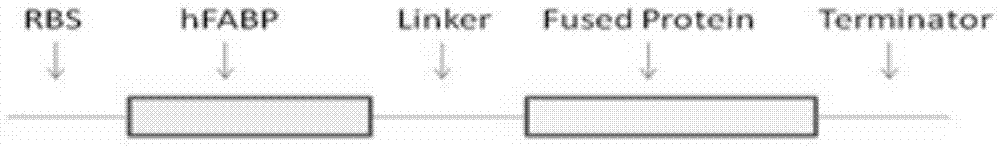
[0030] (3) The full-length coding DNA sequence was synthesized by polymerase chain reaction (PCR), including 5'-6xHisTag (SEQ ID NO.4), hFBP6 amino acid coding sequence, 3'-flexible linker sequence (Linker) and multiple cloning region (SEQ ID NO. 3).
[0031] (4)...
Embodiment 2
[0048] 1. Whole gene synthesis of MT4:
[0049]The synthetic method is a general two-step PCR method (PCR, polymerase chain reaction), and obtains MT4 coding DNA: 1) Overlap extension PCR (OverlapPCR) uses primers as templates to synthesize full-length DNA sequences, and 4 primers (SEQ ID NO: 23-26); 2) using the product as a template to amplify the full-length DNA by PCR, using 2 primers (SEQ ID NO.27-28).
[0050] (1) Head extension PCR parameters: 94°C / 30 seconds, 56°C / 30 seconds, 72°C / 30 seconds, 15 cycles, complement extension 72°C / 2 minutes.
[0051] (2) Full-length synthetic PCR parameters: 94°C / 30 seconds, 58°C / 30 seconds, 72°C / 45 seconds, 30 cycles, complement extension 72°C / 5 minutes.
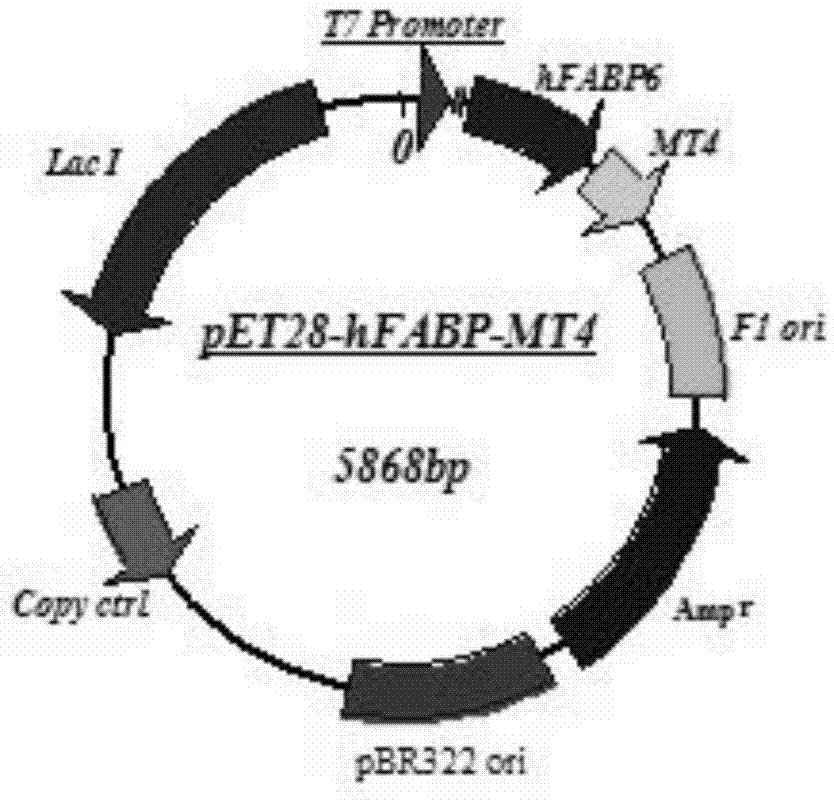
[0052] 2. Construction of hFABP6-MT4 fusion protein expression vector:
[0053] (1) The MT4 DNA sequence obtained by artificial synthesis was purified by KpnI / XhoI double enzyme digestion, electrophoresis, and gel cutting;
[0054] (2) At the same time, the pET28-hFABP6 vector was ...
Embodiment 3
[0058] (1) Construction of the control vector pET28-MT4
[0059] PCR primers for MT4, upstream primer: 5'-TTTTGGTACCATGGATCCGCGCGAATGTGTCTGCATGTCTG-3' (SEQ ID NO.27), downstream primer: 5'-TTTTCTCGAGTTATGGACAGCAGGAGCATTTATC-3' (SEQ ID NO.28).
[0060] The PCR parameters are: 94°C / 30 seconds, 56°C / 30 seconds, 72°C / 30 seconds, 30 cycles.
[0061] (2) Restriction enzyme digestion of MT4 PCR product
[0062] The PCR product was digested with NcoI / XhoI and purified by gel electrophoresis.
[0063] 3. Treat the pET28 vector with NcoI / XhoI double enzyme digestion, electrophoresis and gel purification.
[0064] 4. T4 DNA ligase ligated the linearized pET28 vector and the insert fragment MT4.
[0065] 5. Transform the ligation product into Escherichia coli competent cell BL21(DE3), pick a single colony, amplify and identify the recombinant, and sequence and analyze to obtain the correct pET28-MT4 clone.
[0066] 6. Take the above-mentioned correct clones and culture them in LB medi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com