Stem cell-based medicinal product for treating diabetes and preparing method thereof
A technology of stem cell preparation and diabetes, which is applied in the field of stem cell research, can solve problems such as limited sources, and achieve the effects of abundant sources, convenient collection, and broad application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Preparation of mesenchymal stem cell preparations derived from umbilical cord
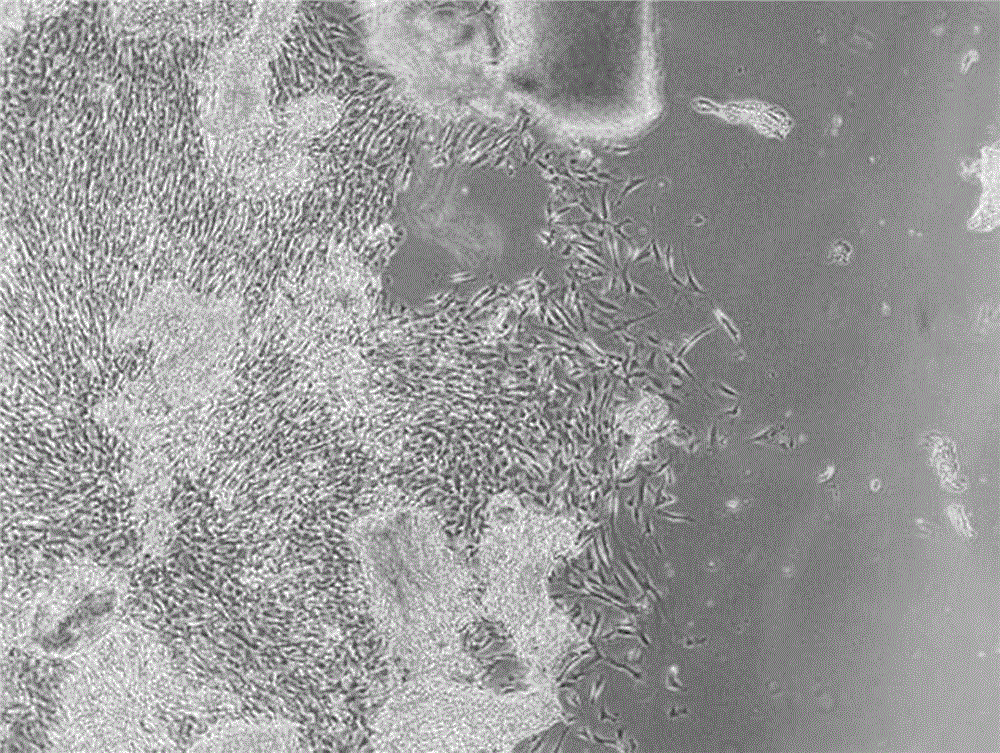
[0032] Operate in the ultra-clean workbench, sterilize the freshly obtained umbilical cord with ethanol, then wash the umbilical cord with saline, separate and strip the blood vessels of the umbilical cord to obtain the Wharton's glue part, cut the Wahton's glue into pieces, and dilute it into a bottle. Use the tissue adherence method to culture in a carbon dioxide incubator, observe the cell growth under a microscope, you can see that the cells grow adherently, are long fusiform, the peritoneum is transparent, the cell body is small, and has a three-dimensional effect, and it is gradually removed by changing the whole volume For non-adherent cells, the primary cells can be harvested when the cell confluence reaches 70-80%. The results are figure 1 ; The obtained primary cells were subcultured in DMEM medium to achieve the purpose of purifying and expanding mesenchymal stem cells. The results ar...
Embodiment 2
[0034] Study on Surface Markers of Mesenchymal Stem Cells Derived from Umbilical Cord
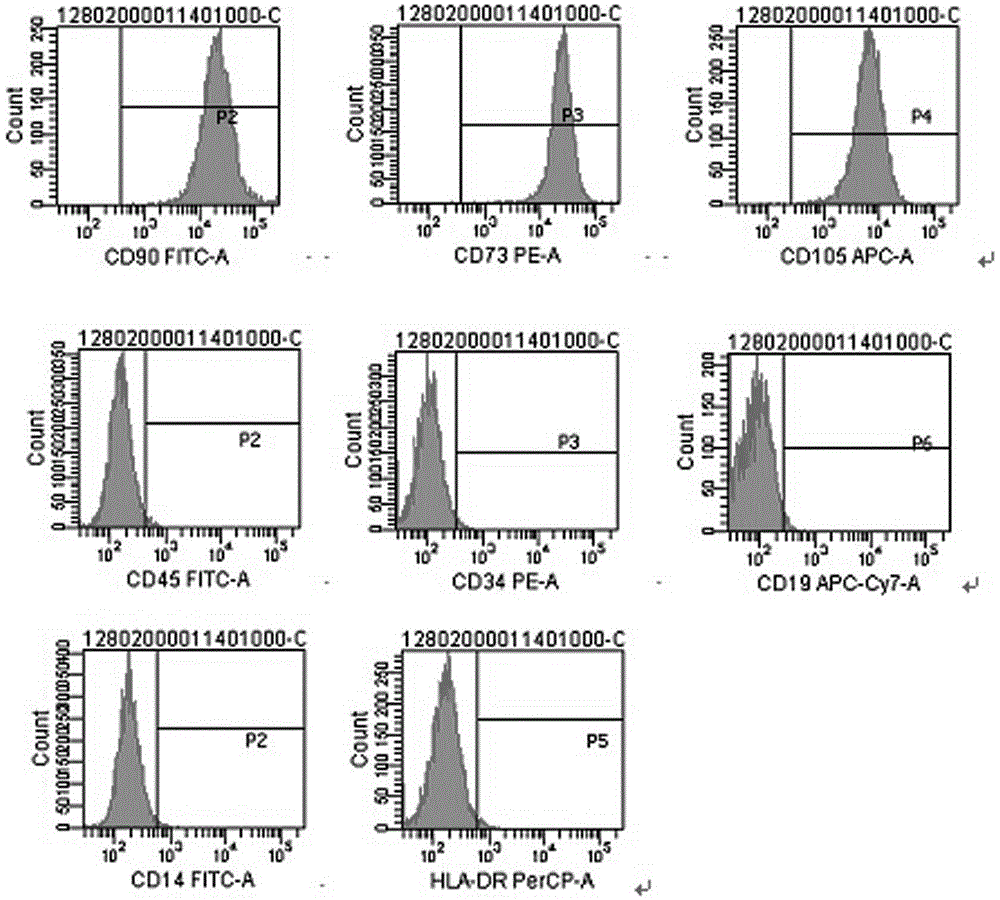
[0035] Use flow cytometry to detect cell surface markers, see the results image 3 . The cell surface markers CD73, CD90, and CD105 related to MSCs are highly expressed, and the expression of each generation is stable, all higher than 95%; cell surface markers CD14, CD34, CD45, CD19 related to hematopoietic cells and related to immune rejection The expression of HLA-DR is low, below 3%. It is proved that MSCs derived from umbilical cord after multiple passages contain stem cells that can proliferate stably. The cells highly express MSCs-related markers, but do not express or low-express hematopoietic cells and cell surface markers related to transplant rejection, suggesting that such MSCs cells are a type of low Immunogenic stem cells may play a role in fighting autoimmune diseases and treating rejection after transplantation.
Embodiment 3
[0037] Proliferation of mesenchymal stem cells derived from umbilical cord
[0038] The passage cells were planted in a 96-well plate, divided into 12 groups, each with 7 wells, and 200 μl of cell suspension was added to each well. Randomly take subcultured cells, detect the OD value of each experimental group and blank control group according to the MTT microplate reader, take the average, and draw the stem cell growth curve. The results are shown in Figure 4 . Experimental results show that mesenchymal stem cells can proliferate normally.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com