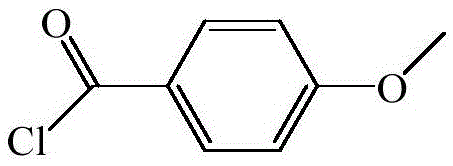
Preparation method of p-anisoyl chloride
A technology of methoxybenzoyl chloride and methoxybenzoic acid, applied in the field of organic synthesis, can solve the problems of unsuitability for industrial production, high reaction conditions, long reaction time, etc., and achieves easy operation, high purity and mild reaction. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Add 15.23g (0.1mol) of p-methoxybenzoic acid, 30.5g of dichloroethane, and 0.3g (0.004mol) of DMF into the reaction vessel of the tail gas absorption device equipped with water, raise the temperature to 40°C, and slowly drop Add BTC-C with a concentration of 2mol / L 2 h 4 Cl 2 The solution was 15.5ml, and after the dropwise addition, the reaction was refluxed for 1 hour. After the solvent was recovered by distillation under reduced pressure, 16.88g of p-methoxybenzoyl chloride was obtained, the content was 99.62% (gas chromatography), and the yield was 98.57%.
Embodiment 2
[0021] Add 15.23g (0.1mol) of p-methoxybenzoic acid, 45.7g of dichloroethane, and 0.48g (0.006mol) of pyridine into the reaction vessel of the tail gas absorption device equipped with water, raise the temperature to 42°C, and slowly drop Add BTC / C with a concentration of 2mol / L 2 h 4 Cl 2 The solution was 15.5ml, and after the dropwise addition, the reaction was refluxed for 1.5 hours. After the solvent was recovered by distillation under reduced pressure, 16.86g of p-methoxybenzoyl chloride was obtained, the content was 99.68% (gas chromatography), and the yield was 98.28%.
Embodiment 3
[0023] Add 15.23g (0.1mol) of p-methoxybenzoic acid, 60.9g of dichloroethane, and 0.38g (0.005mol) of DMF into the reaction vessel of the tail gas absorption device equipped with water, raise the temperature to 45°C, and slowly drop Add BTC / C with a concentration of 2mol / L 2 h 4 Cl 2 The solution was 15.5ml, and after the dropwise addition, the reaction was refluxed for 2 hours. After the solvent was recovered by distillation under reduced pressure, 16.92g of p-methoxybenzoyl chloride was obtained, with a content of 99.87% (gas chromatography), and a yield of 98.82%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com