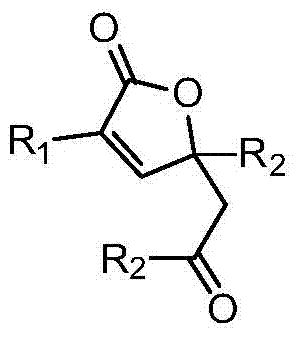
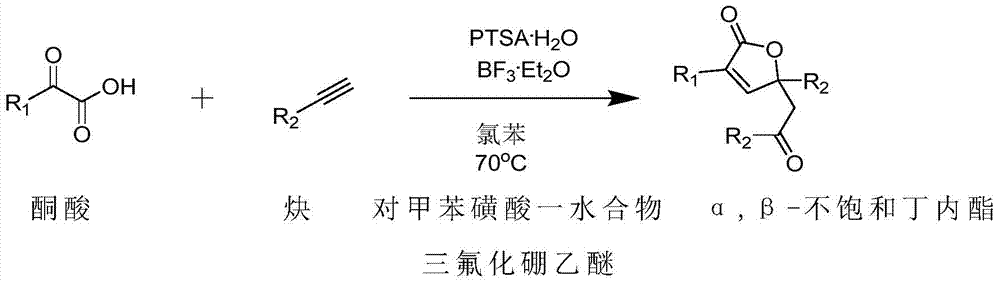
Method for preparing α,β-unsaturated butyrolactone from keto acid and alkyne
A technology of unsaturated butyrolactone, which is applied in the direction of organic chemistry, can solve the problems of high requirements for the reaction environment and complicated operation, and achieve the effect of simple operation, low price and improved economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
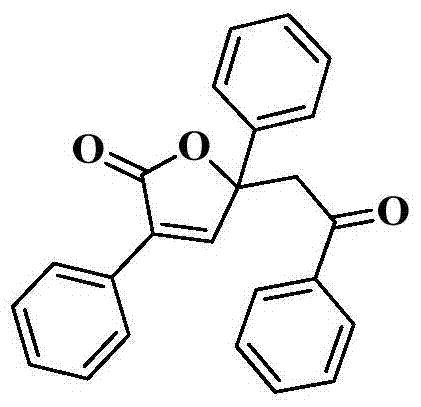
[0025] Example 1 A compound was synthesized by using the reaction of benzoylformic acid and phenylacetylene. Its molecular formula is:
[0026]
[0027] Add 0.3mmol benzoylformic acid, 0.9mmol phenylacetylene, 0.6mmol p-toluenesulfonic acid monohydrate, 0.06mmol boron trifluoride ether and 2mL chlorobenzene to a reaction tube at one time, react at 70°C for 6h, and the reaction is completed Afterwards, the solvent was evaporated under reduced pressure, and petroleum ether:ethyl acetate was used for column chromatography. The volume ratio of petroleum ether:ethyl acetate was 10:1, and then the pure product was obtained with a yield of 78% and a purity of 98%.
[0028] After NMR analysis: 1 H NMR (400MHz, CDCl 3 )δ8.35(s,1H),7.92-7.86(m,4H),7.59-7.53(m,3H),7.46-7.30(m,8H),4.13(d,J=16.4Hz,1H),3.70 (d,J=16.4Hz,1H). 13 C NMR (100MHz, CDCl 3 )δ 195.1, 169.8, 149.9, 138.2, 136.0, 133.2, 130.1, 129.0, 128.8, 128.3, 128.2, 128.2, 128.1, 127.8, 126.8, 125.3, 85.7, 48.2.
Embodiment 2
[0029] Example 2 A compound was synthesized by using the reaction of p-methoxybenzoylformic acid and phenylacetylene. Its molecular formula is:
[0030]
[0031] Add 0.3mmol p-methoxybenzoylformic acid, 0.9mmol phenylacetylene, 0.6mmol p-toluenesulfonic acid monohydrate, 0.06mmol boron trifluoride diethyl ether and 2mL chlorobenzene in one reaction tube, and react at 70°C 9h, after the reaction was completed, the solvent was evaporated under reduced pressure, and petroleum ether: ethyl acetate column chromatography was used. The volume ratio of petroleum ether: ethyl acetate was 10:1, and then the pure product was obtained with a yield of 88% and a purity of 98.5 %.
[0032] After NMR analysis: 1 H NMR (400MHz, CDCl 3 )δ8.23(s,1H),7.91-7.82(m,4H),7.58-7.51(m,3H),7.46-7.40(m,2H),7.39-7.34(m,2H),7.33-7.28( m,1H),6.92(d,J=8.8Hz,2H),4.12(d,J=16.4Hz,1H),3.81(s,3H),3.67(d,J=16.4Hz,1H). 13 C NMR (100MHz, CDCl 3 )δ 195.7, 170.6, 160.5, 148.1, 138.9, 136.6, 133.7, 129.9, 128.8...
Embodiment 3
[0033] Example 3 Compounds were synthesized by using the reaction of p-methoxybenzoylformic acid and p-methylphenylacetylene. Its molecular formula is:
[0034]
[0035] Add 0.3mmol p-methoxybenzoylformic acid, 0.9mmol p-toluene acetylene, 0.6mmol p-toluenesulfonic acid monohydrate, 0.06mmol boron trifluoride diethyl ether and 2mL chlorobenzene in one reaction tube, at 70 Reaction at ℃ for 13 hours, after the reaction was completed, the solvent was evaporated under reduced pressure, and petroleum ether: ethyl acetate column chromatography was used. The volume ratio of petroleum ether: ethyl acetate was 10:1, and then the pure product was obtained with a yield of 81%. , 98% purity.
[0036] After NMR analysis: 1 H NMR (400MHz, CDCl 3 )δ8.22(s,1H),7.89-7.83(m,2H),7.79(d,J=8.0Hz,2H),7.42(d,J=8.0Hz,2H),7.23(d,J=8.0 Hz,2H),7.17(d,J=8.0Hz,2H),6.95-6.89(m,2H),4.10(d,J=16.4Hz,1H),3.82(s,3H),3.62(d,J =16.4Hz,1H),2.39(s,3H),2.32(s,3H). 13 C NMR (100MHz, CDCl 3 )δ 194.8, 170.1,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com