Method for synthesizing and secreting black widow spider traction silk protein 2 through silkworm silkgland bioreactor
A technology of pulling silk protein and bioreactor, which is applied in the field of synthesizing and secreting black widow spider pulling silk protein 2, can solve the problems of lack of spider silk and no expression of spider pulling silk protein 2, etc., and achieve the effect of improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
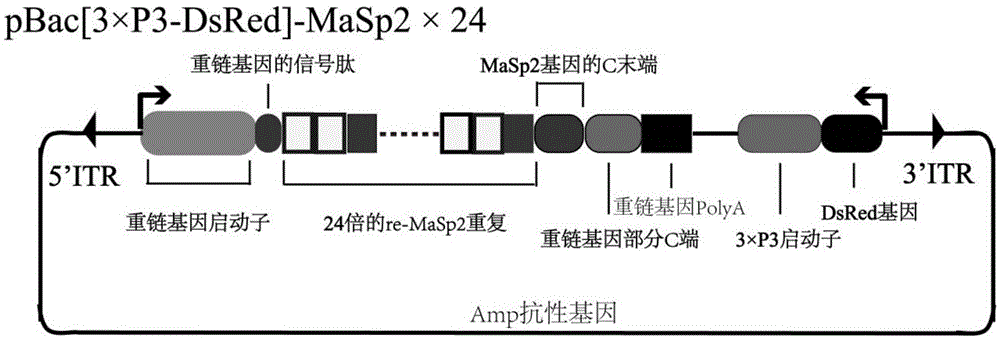
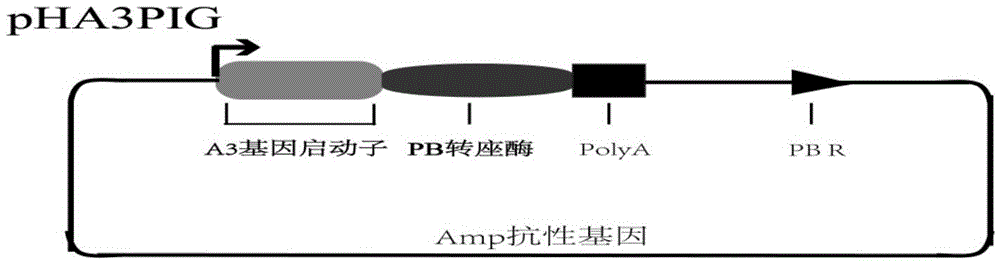
[0043] The above constructed pBac[3xP3-DsRed]-MaSp2×24 plasmid ( figure 1 ) and the helper plasmid pHA3PIG plasmid that can provide piggyBac transposase ( figure 2 ) were mixed at a ratio of 1:1, the total concentration of the two plasmids was 0.4 μg / μl, the plasmids were dissolved in pH=7, 0.5 mM phosphate buffer, and then introduced into the silkworm within 6 hours after laying eggs by microinjection. In fertilized eggs, the total volume of introduction is 10nl. The microinjected silkworm eggs are reared to adults under the condition of 25° C. and 85% humidity, and crossed with non-transgenic silkworms for passage, which is the G1 generation. During the turning green stage of the G1 generation eggs in the transgenic experiment, a transgenic silkworm moth expressing the DsRed marker gene was observed and obtained through a fluorescence microscope (Olympus, SZX12, Japan). The transgenic silkworms from the G2 generation onwards are all reared with single moths, and the trans...
Embodiment 2
[0050] The above constructed pBac[3xP3-DsRed]-MaSp2×24 plasmid ( figure 1 ) and the helper plasmid pHA3PIG plasmid that can provide piggyBac transposase ( figure 2 ) were mixed at a ratio of 1:1, the total concentration of the two plasmids was 0.4 μg / μl, the plasmids were dissolved in pH=7, 0.5 mM phosphate buffer, and then introduced into the silkworm within 6 hours after laying eggs by microinjection. In fertilized eggs, the total volume of introduction is 10nl. The microinjected silkworm eggs are reared to adults under the condition of 25° C. and 85% humidity, and crossed with non-transgenic silkworms for passage, which is the G1 generation. During the turning green stage of the G1 generation eggs in the transgenic experiment, the transgenic silkworm 2 moths expressing the DsRed marker gene were obtained by observation with a fluorescence microscope (Olympus, SZX12, Japan), and the silkworms were reared until the adults were hybridized with non-transgenic silkworms, which...
Embodiment 3
[0057] The above constructed pBac[3xP3-DsRed]-MaSp2×24 plasmid ( figure 1 ) and the helper plasmid pHA3PIG plasmid that can provide piggyBac transposase ( figure 2 ) were mixed at a ratio of 1:1, the total concentration of the two plasmids was 0.4 μg / μl, the plasmids were dissolved in pH=7, 0.5 mM phosphate buffer, and then introduced into the silkworm within 6 hours after laying eggs by microinjection. In fertilized eggs, the total volume of introduction is 10nl. The microinjected silkworm eggs are reared to adults under the condition of 25° C. and 85% humidity, and crossed with non-transgenic silkworms for passage, which is the G1 generation. During the turning green stage of the G1 generation eggs in the transgenic experiment, a transgenic silkworm moth expressing the DsRed marker gene was observed and obtained through a fluorescence microscope (Olympus, SZX12, Japan). The transgenic silkworms from the G2 generation onwards are all reared with single moths, and the trans...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com