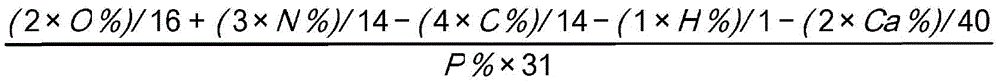Flame retardant prepared from amide derivatives and process for making the same
A technology of derivatives and mixtures, applied in the field of flame retardants prepared from amide derivatives and their manufacture, can solve the problems of unwanted moisture/moisture absorption, loss of flame retardant properties, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
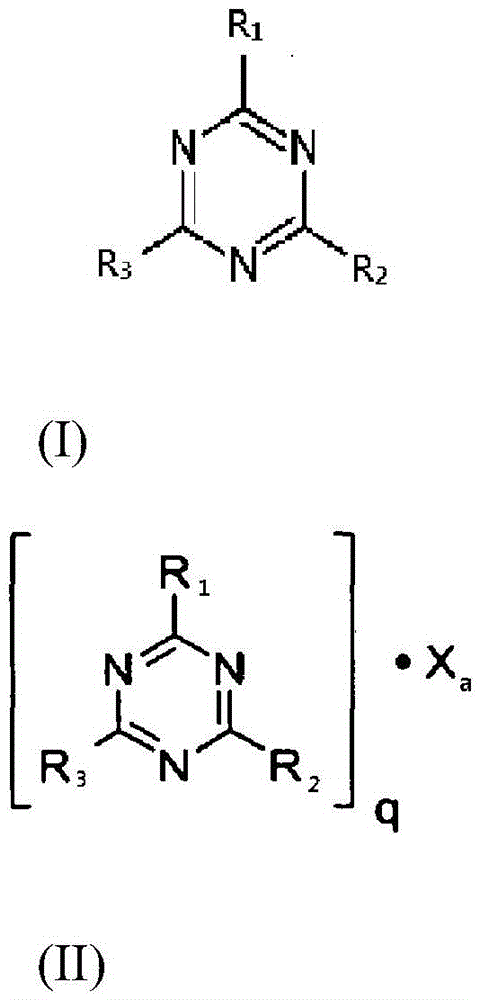
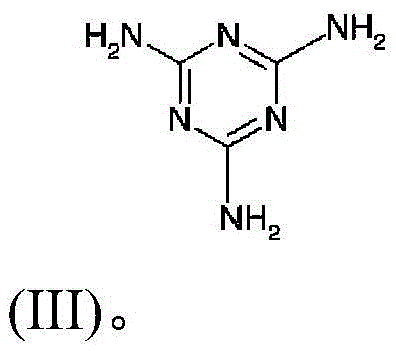
[0074] With stirring, 78 g of H 3 PO 3 Add 70.86g of Ca(OH) 2 and 400g of H 2 O mixture. Subsequently, the resulting mixture was stirred at 40° C. for 2.5 h, and then filtered to obtain a solid mixture containing calcium phosphite. The solid mixture was washed with deionized water and dried at 105 °C for 3 h.
[0075] A 250 mL reactor equipped with a mechanical stirrer was charged with 120 g of water and 30.46 g of the calcium phosphite mixture obtained as mentioned above. Under stirring, 53.3 g of an 85% orthophosphoric acid hydrochloric acid solution were added to the resulting mixture at room temperature. After the addition, the mixture was stirred for half an hour and then filtered to remove insoluble solids to give a clear solution. Then 58 g of melamine were slowly added to the filtrate under stirring for another 1 hour of reaction. After this time, the mixture was evaporated to remove all water to obtain a white solid. The white solid was heated at 330° C. for 3 h...
example 2
[0080] A 500 mL reactor equipped with a mechanical stirrer was charged with 400 g of water and 60 g of calcium phosphite mixture. Under stirring, 91.34 g of a 38% hydrochloric acid solution were added to the resulting mixture at room temperature. After the addition, the mixture was stirred for half an hour and then filtered to remove insoluble solids to give a clear solution. Then 144 g of melamine were slowly added to the filtrate with stirring for another 1 hour of reaction. After this time, the reaction mixture was filtered to remove the aqueous solution and a white solid was obtained. This white solid was then heated at 330° C. for 3 hours (with a weight loss of 23%) to convert it to the calcium melamine phosphate salt. By elemental analysis, the calcium melamine phosphate salt thus obtained contained 41.55% of N, 7.27% of P and 7.98% of Ca. By calculation, the percentage of oxygen element remaining in the calcium melamine phosphate salt is 23.67% by weight. Therefore,...
example 3
[0083] A 500 mL reactor equipped with a mechanical stirrer was charged with 400 g of water and 65.5 g of calcium phosphite mixture. Under stirring, 91.34 g of a 38% hydrochloric acid solution were added to the resulting mixture at room temperature. After the addition, the mixture was stirred for half an hour and then filtered to remove insoluble solids to give a clear solution. Then 144 g of melamine were slowly added to the filtrate with stirring for another hour of reaction. After this time, the mixture was filtered to remove the aqueous solution to obtain a white solid. This white solid was heated at 330°C for 3 hours to yield calcium melamine phosphate salt with 23% weight loss during heating. The calcium melamine phosphate salt thus obtained contained 31.5% of N, 16.0% of C, 2.60% of H, 14.2% of P and 7.98% of Ca by elemental analysis. By calculation, the percentage of oxygen element remaining in the calcium melamine phosphate salt is 27.72% by weight. Thus, based on ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com