Method for extracting lithium isotopes
A lithium isotope and extraction technology, applied in the separation of different isotopic elements, separation methods, chemical instruments and methods, etc., can solve the problems of limiting the reusability of resin materials, achieve good lithium isotope separation efficiency, improve stability, and avoid Human health hazards and effects of environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
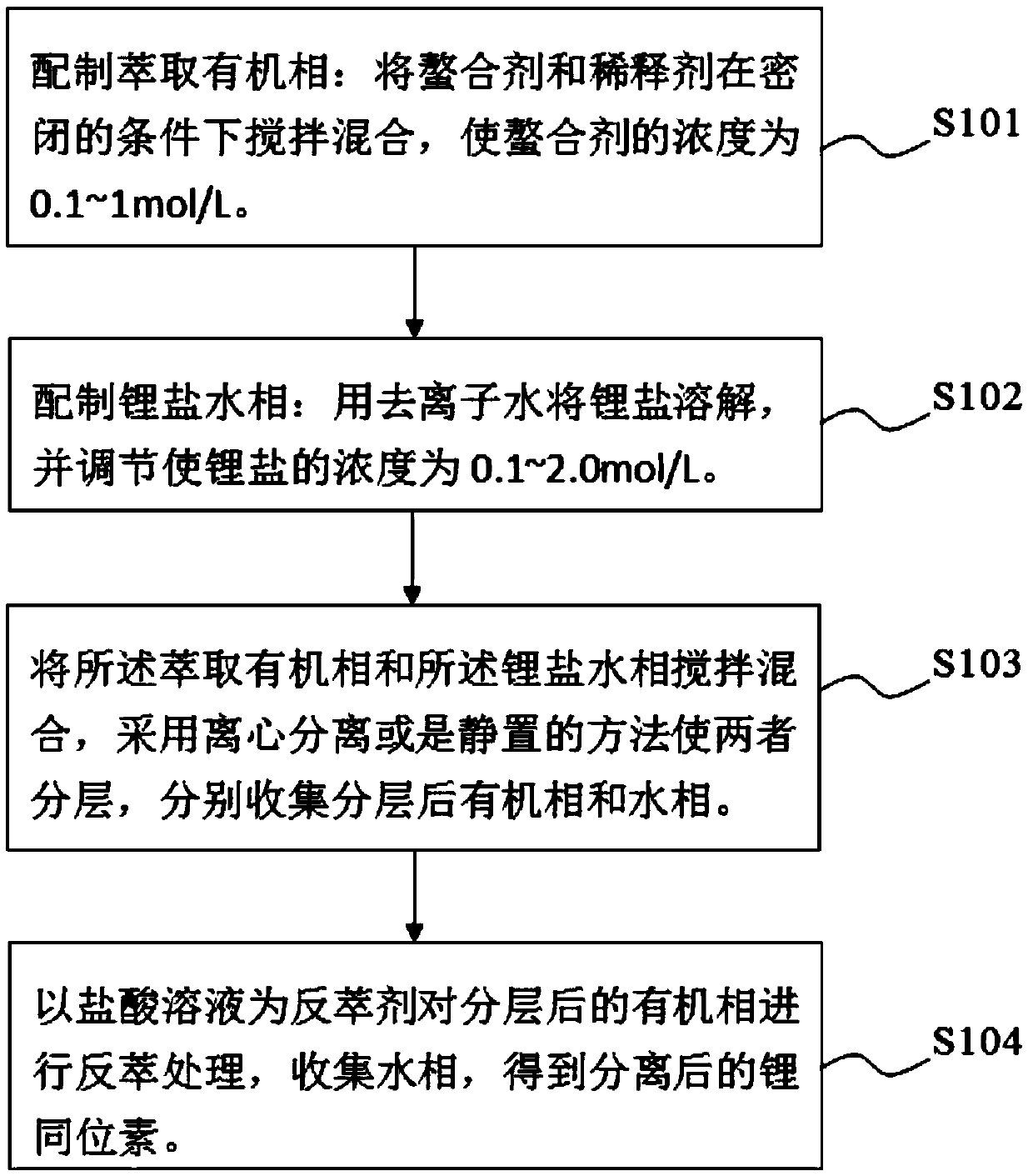
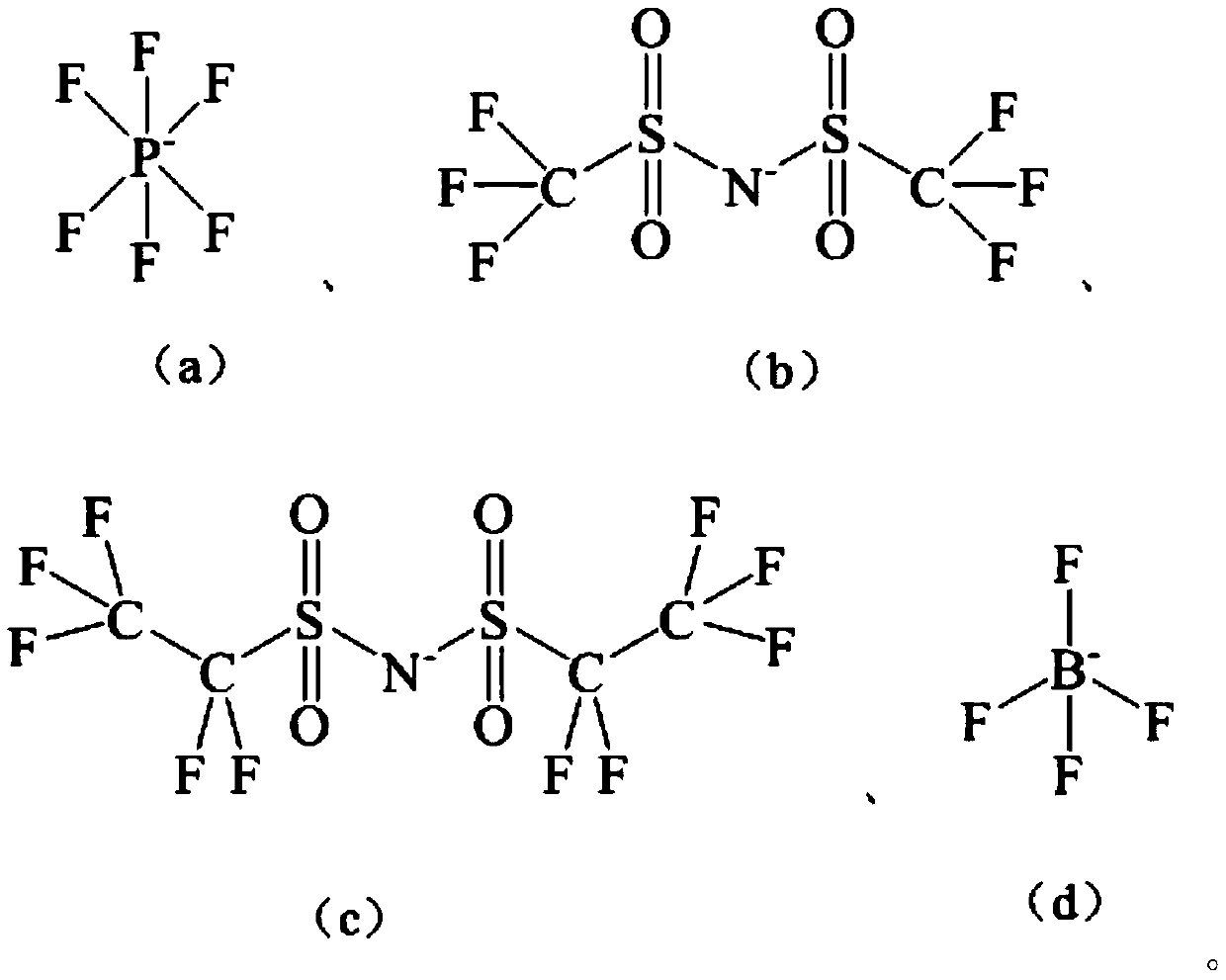
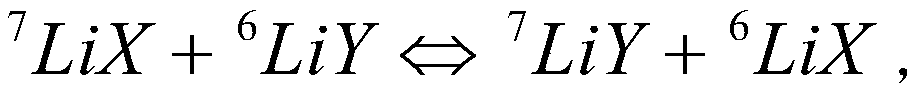
[0049] In a 100mL separatory funnel, add 20mL of aqueous phase (0.2mol / L lithium thiocyanate) and 40mL of organic phase (0.1mol / L hydrophobic ionic liquid containing crown ether structure: 1-allyl-3 -(6'-Oxo-benzo-15-crown-5-hexyl)imidazole hexafluorophosphate, abbreviated as [A(Benzo15C5)HIM][PF 6 ], the diluent is chloroform), violently shaken on the Conrad shaker for 10min, centrifuged to separate the aqueous phase and the organic phase, and collected the organic phase. The extraction rate of a single lithium was tested to be 26.1%. Use 20 mL of 0.2 mol / L HCl solution for the organic phase, place it on a Consonian shaker for 20 min, and centrifuge to collect the aqueous phase. The organic phase is directly used for stripping, and the operation is repeated 5 times, and the stripping yields of 1 and 5 operations of lithium are respectively 27.4% and 99.7%, and the isotope separation coefficient α ( 6 Li / 7 Li) is 1.039.
Embodiment example 2
[0051] In a 100mL separatory funnel, add 20mL of aqueous phase (0.5mol / L lithium trifluoromethyl acetate) and 20mL of organic phase (0.4mol / L hydrophobic ionic liquid containing crown ether structure: 1-allyl -3-(6'-Oxy-benzo-15-crown-5-hexyl)imidazole bistrifluoromethylsulfonimide salt, abbreviated as [A(Benzo15C5)HIM][N(SO 2 CF 3 ) 2 ], the diluent is dichlorobenzene), violently oscillating 20min on a Conrad shaker, centrifuging the aqueous phase and the organic phase, collecting the organic phase, and testing to obtain a single lithium extraction rate of 23.2%. Use 24 mL of 0.5 mol / L HCl solution for the organic phase, place it on a Consonian shaker for 30 min, and centrifuge to collect the aqueous phase. The organic phase is directly used for stripping, and the operation is repeated 5 times, and the stripping yields of 1 and 5 operations of lithium are respectively 31.4% and 99.2%, and the isotope separation coefficient α ( 6 Li / 7 Li) was 1.041.
Embodiment 3
[0053] In a 100mL separatory funnel, add 40mL of aqueous phase (0.8mol / L lithium thiocyanate) and 20mL of organic phase (1.0mol / L hydrophobic ionic liquid containing crown ether structure: 1-allyl-3 -(6'-Oxo-benzo-15-crown-5-hexyl)imidazole hexafluorophosphate, abbreviated as [A(Benzo15C5)HIM][PF 6 ], the diluent is dichlorobenzene), violently oscillating 30min on a Conrad shaker, centrifuging the aqueous phase and the organic phase, collecting the organic phase, and testing to obtain a single lithium extraction rate of 21.7%. Use 20 mL of 1.0 mol / L HCl solution for the organic phase, place it on a Consonian shaker for 45 minutes, and centrifuge to collect the aqueous phase. The organic phase is directly used for stripping, and the operation is repeated 5 times, and the stripping yields of 1 and 5 operations of lithium are respectively 27.9% and 99.5%, and the isotope separation coefficient α ( 6 Li / 7 Li) was 1.043.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com