Heliotropin preparation method
A technology of jasmonal and acetic acid aqueous solution, applied in organic chemistry and other fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
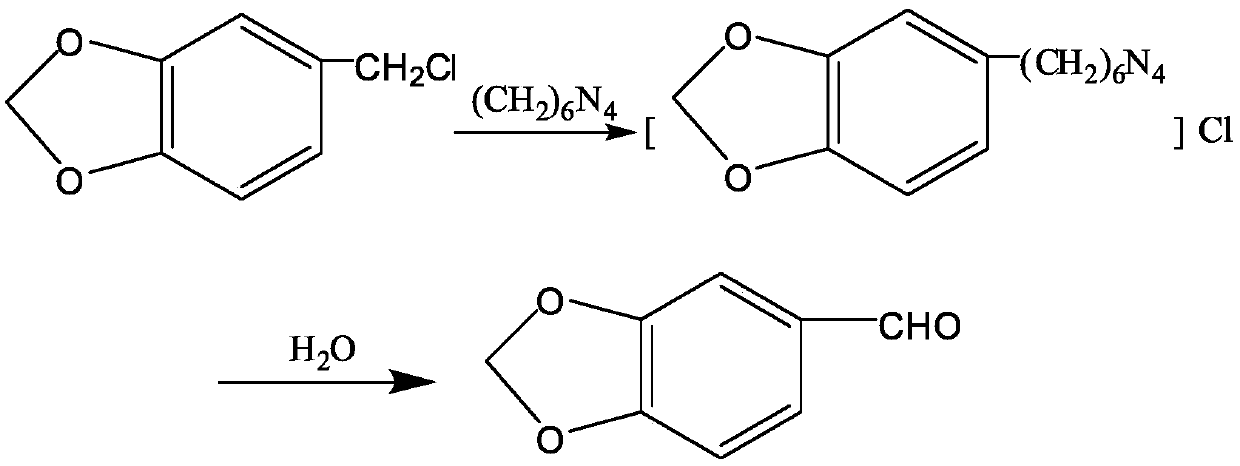
[0015] Add 25ml of glacial acetic acid and 25ml of water into the reaction bottle, start stirring to configure a 50% acetic acid aqueous solution, add urotropine at one time, the amount of urotropine added is shown in Table 1, and when the water bath is cooled to 30°C, add 3 , 4-methylenedioxybenzyl chloride 68g, after the end, keep warm at 10~40°C for 4 hours; add 400ml pure water to the reactant and reflux for 2.5 hours; cool to 30-40°C and extract with chloroform, combine the organic layers to reduce The concentrate was concentrated under reduced pressure, and the concentrate was distilled under reduced pressure, and the fraction with T=85~105°C and P=70~150Pa was collected to obtain white jasmonal crystals. The content and yield are shown in Table 1.
[0016] Table 1: The selection of different addition amounts of urotropine and the content and yield of jasmonal
[0017] serial number Addition of urotropine Molar ratio of 3,4-methylenedioxybenzyl chloride to ...
Embodiment 2
[0019] Add 25ml of glacial acetic acid and 25ml of water into the reaction bottle, start stirring to configure a 50% aqueous solution of acetic acid, add 111.8g of urotropine at one time, and add 3,4-methylenedioxybenzyl dropwise when the water bath is cooled to 30°C Chlorine 68g, keep warm at 10-40°C after the end, see Table 2 for the selection of the holding time; add 400ml pure water to the reactant and reflux for 2.5 hours; cool to 30-40°C and extract with chloroform, combine the organic layers and concentrate under reduced pressure , the concentrate was distilled under reduced pressure, and the fractions with T=85~105°C and P=70~150Pa were collected to obtain white jasmonal crystals. The contents and yields are shown in Table 2.
[0020] Table 2: The selection of different holding times and the content and yield of jasmonal
[0021] serial number Holding time content% Molar yield % 1 2 hours 98.12 69.61 2 3 hours 98.67 72.15 3 4 hours...
Embodiment 3
[0023] Add 25ml of glacial acetic acid and water into the reaction bottle, start stirring to configure an acetic acid aqueous solution, the concentration of the acetic acid aqueous solution is shown in Table 3, add 111.8g of urotropine at one time, and add 3,4-methylene dropwise when the water bath is cooled to 30°C Dioxybenzyl chloride 68g, heat preservation at 10-40°C for 4 hours after the end; add 400ml pure water to the reactant and reflux for 2.5 hours; cool to 30-40°C and extract with chloroform, combine the organic layers and concentrate under reduced pressure, the concentrate Carry out vacuum distillation, collect the distillate of T=85~105 ℃, P=70~150Pa, the content and yield of white jasmonal crystals are shown in Table 3.
[0024] Table 3: the selection situation of acetic acid aqueous solution content and the content and yield of jasmonal
[0025] serial number Acetic acid / water Acetic acid aqueous solution content content% Molar yield % 1 1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com