Method for evaluating genetic toxicity of drinking water through multi-genetic-end point biological group tests
A genotoxicity, drinking water technology, applied in the direction of testing water, material inspection products, measuring devices, etc., can solve the problems of drinking water safety misjudgment, false negative, affecting the quality of human gene pool, etc., to achieve good versatility and detection means easy effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
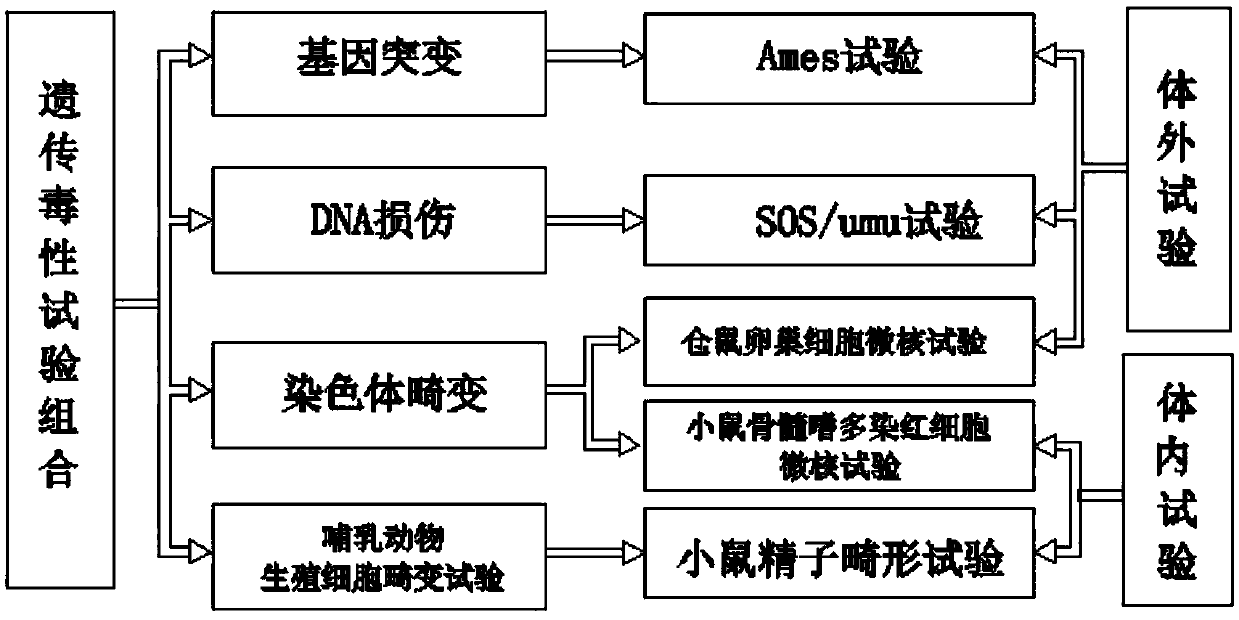
[0015] Specific implementation mode 1: This implementation mode provides a method covering multiple genetic endpoints to evaluate the genotoxicity of drinking water, and its technical measures adopt the following scheme:
[0016] Although there are many methods for detecting genotoxicity at present, they can be roughly divided into the following three categories in terms of detection methods: gene mutation test, chromosome aberration test, and DNA damage and repair test. The selection of mutagenesis tests should meet the following principles: ①It should include multiple genetic endpoints; ②Test organisms include prokaryotic, eukaryotic and mammalian species; ③Include in vivo and in vitro tests; ④Include sex cells and somatic cells; ⑤Detection methods Relatively simple, fast, economical and good laboratory versatility and other principles. In addition, the weight value of the mutagenic test evaluation results follows the following principles: (1) The degree of evolution of the ...
specific Embodiment approach 2
[0033] Specific implementation mode 2: In this implementation mode, a waterworks in the south of China is evaluated. The effluent from the filter of the water intake plant and the disinfected water were evaluated for genotoxicity according to the method described in Embodiment 1.
[0034] 1. Ames test analysis
[0035] The histidine-deficient Salmonella typhimurium (TA98 and TA100 strains) used in this test were directly provided by the United States University of California Ames laboratory, and the characteristics of the identified strains met the test requirements.
[0036] Count the number of back-changing colonies in each plate, and calculate the mean number and standard deviation of 6 parallel plates to obtain the mutagenic rates MR of TA98 and TA100 respectively. The results are shown in Table 1. It is generally believed that the criteria for judging a positive Ames test (gene mutation that can cause base substitution type) must meet two conditions at the same time: ① T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Graininess | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com