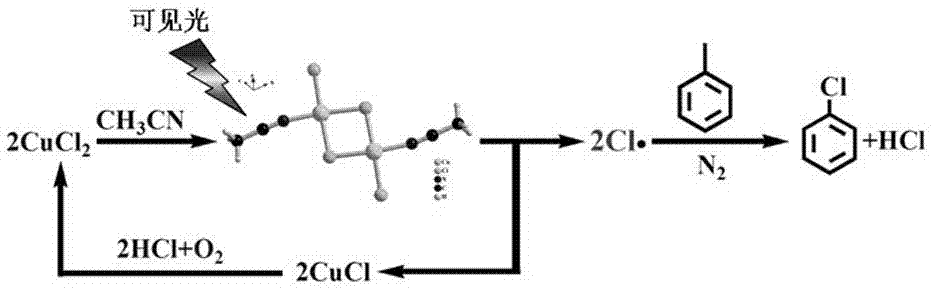
A kind of method that photocatalytic toluene is converted into chlorinated toluene
A chlorotoluene, photocatalytic technology, applied in chemical instruments and methods, organic chemistry, halogenated hydrocarbon preparation, etc., can solve the problem of product use limitation, increase impurity residue, affect product purity and other problems, achieve mild experimental conditions, selectable The effect of improving the performance and promoting the application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
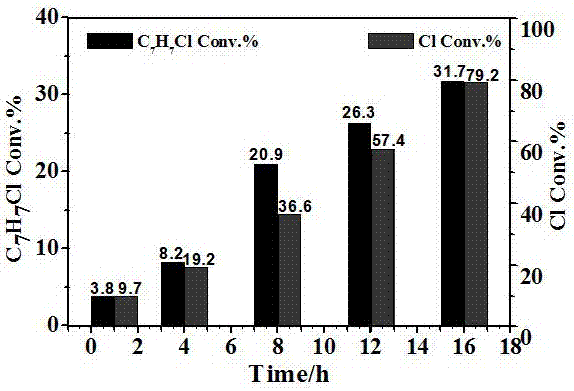
[0023] The effect of different times on the response
[0024] 1) 0.1mmol CuCl 2 , 1 mL of acetonitrile, 100 μL of toluene; add copper chloride to acetonitrile, stir at room temperature for 1 min, first prepare the copper chloride acetonitrile complex; then add toluene to it to obtain a toluene solution;
[0025] 2) Put the toluene solution obtained in step (1) into an atmospheric pressure batch reaction device. After sealing, use a vacuum pump to remove the air in the reactor, and then fill it with nitrogen to ensure the anaerobic condition of the reaction. Stir magnetically at room temperature ; The light intensity of the visible light reaction is 300 mW / cm -2 (Wavelength is 420~780 nm);
[0026] 3) After the reaction is complete, the reactants and products are detected by GC-MS 6890N mass spectrometer. Quantitative analysis with 6890N chromatograph FID detector. The chromatographic column is HP-5 (5% polymethylsilane fixed solution, the specification is 30 m × 0.32 mm ×...
Embodiment 2
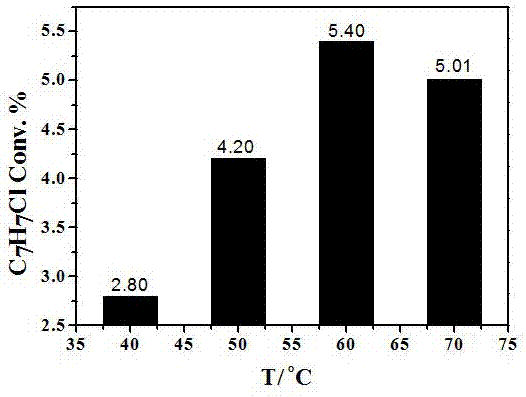
[0029] Experimental results at different reaction temperatures (1h)
[0030] 1) 0.1mmol CuCl 2 , 1 mL of acetonitrile, 100 μL of toluene; add copper chloride to acetonitrile, stir at room temperature for 1 min, first prepare the copper chloride acetonitrile complex; then add toluene to it to obtain a toluene solution;
[0031] 2) Put the toluene solution obtained in step (1) into an atmospheric pressure batch reaction device. After sealing, use a vacuum pump to remove the air in the reactor, and then fill it with nitrogen to ensure the anaerobic condition of the reaction. Stir magnetically at room temperature ; The light intensity of the visible light reaction is 300 mW / cm -2 (Wavelength is 420~780 nm);
[0032] 3) After the reaction is complete, the reactants and products are detected by GC-MS 6890N mass spectrometer. Quantitative analysis with 6890N chromatograph FID detector. The chromatographic column is HP-5 (5% polymethylsilane fixed solution, the specification is 3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com