Preparation method of 3, 5-dibromo iodobenzene
A technology of dibromoiodobenzene and iodoaniline, applied in 3 fields, can solve problems such as many by-products, high toxicity, long reaction steps, etc., and achieves the effects of improving yield, easy operation and control, and being beneficial to industrialized production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] 3 of the present invention, the preparation method of 5-dibromoiodobenzene comprises the following steps:
[0021] Step 1, the preparation step of 2,6-dibromo-4-iodoaniline intermediate, add p-iodoaniline and absolute ethanol in a container, under normal temperature conditions, add dibromohydantoin in batches; no raw materials are detected by HPLC When the product is produced, stop the reaction; directly filter to obtain the 2,6-dibromo-4-iodoaniline intermediate; the molar ratio of p-iodoaniline to dibromohydantoin is 1:1.
[0022] Step 2, add 2-, 6-dibromo-4-iodoaniline intermediate and hypophosphorous acid in another container, add sodium nitrite solution dropwise after cooling down, a large amount of solids will wash out after dropping, filter directly , recrystallized from absolute ethanol to obtain needle-like 3,5-dibromoiodobenzene. The molar ratio of 2,6-dibromo-4-iodoaniline intermediate to sodium nitrite is 1:1.
[0023] Preparation of intermediate 2,6-dibro...
Embodiment 2
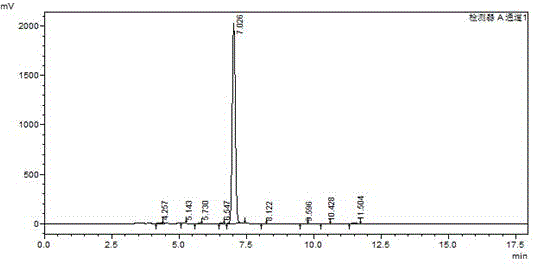
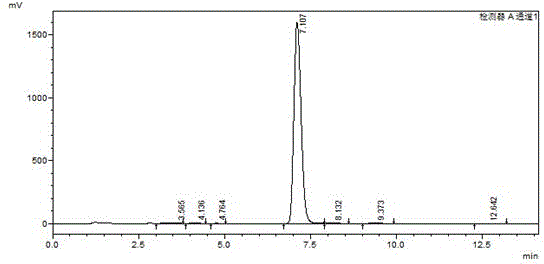
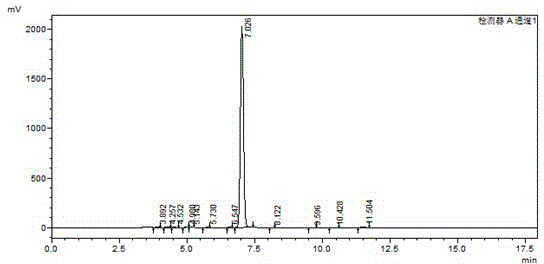
[0030] The preparation of intermediate 2,6-dibromo-4-iodoaniline: the detailed steps are as follows: wherein, the dosage is as follows: p-iodoaniline: 219g, dibromohydantoin: 290g, absolute ethanol: 1800ml. Add 219g of p-iodoaniline (1mol) and 1000ml of absolute ethanol into a 2000ml reaction bottle, stir for 20 minutes to disperse evenly. Then add 290 g of dibromohydantoin in batches, control the reaction temperature at 20-25° C., and complete the addition in about 2 hours. After the addition, a large amount of white solids will precipitate out. Reaction was continued at this temperature for 1 hour. The reaction was monitored by HPLC. When the raw materials disappeared, the reaction was stopped, filtered at 20-25°C, and dried under normal pressure at 100°C to obtain 350 g of off-white powdery solid. like image 3 And the HPLC collection of illustrative plates of 2,6-dibromo-4-iodoaniline shown in Table 3, collection of illustrative plates shows 2,6-dibromo-4-iodoaniline con...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com