Synthetic method for precursor of anthraquinone reactive disperse dye used in supercritical CO2
A technology of reactive disperse dyes and synthesis methods, which is applied in the field of synthesis of anthraquinone-type reactive disperse dye precursors for supercritical CO2, and can solve the problems of poor applicability and few types of reactive disperse dyes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
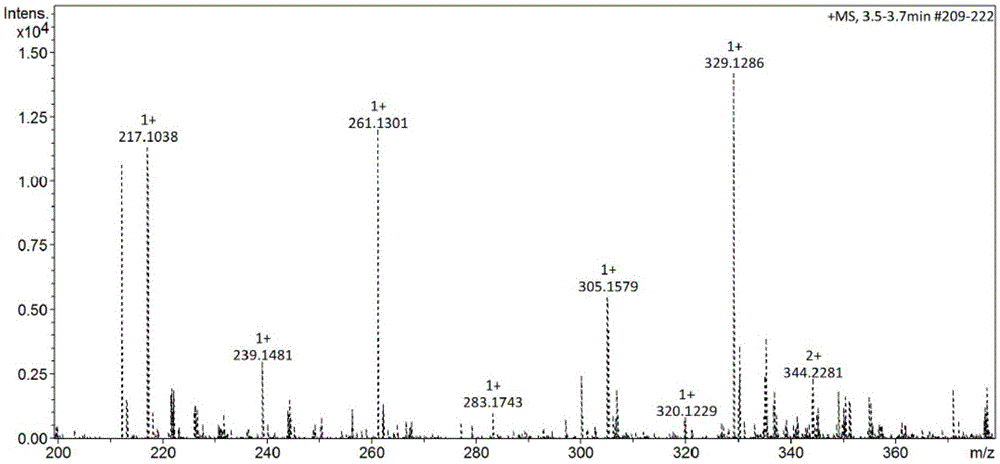
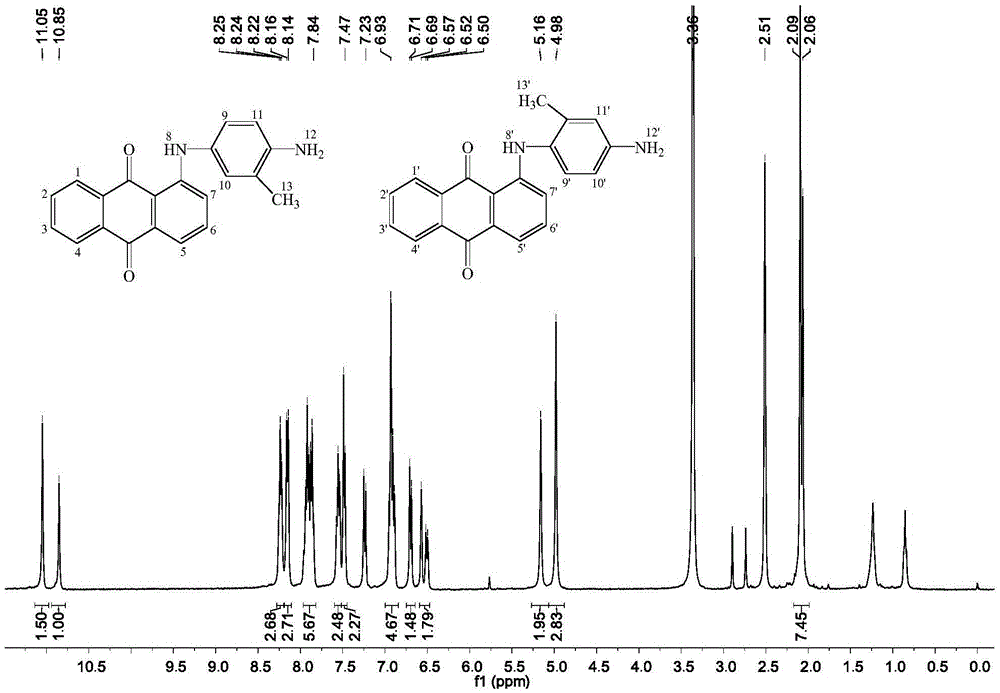
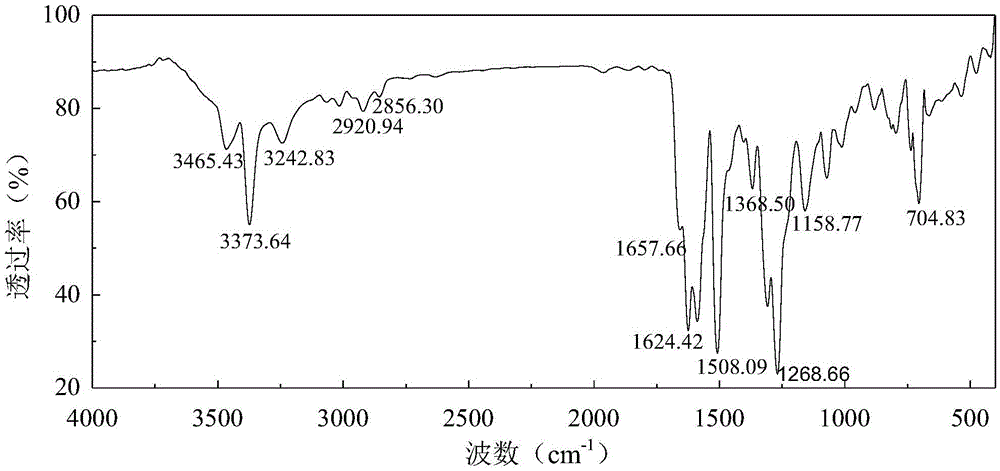
[0022] Supercritical CO 2 With the synthetic method of anthraquinone type reactive disperse dye precursor, comprise the steps:
[0023] (1) Add 0.005mol2,5-diaminotoluene sulfate and 0.0075molK 2 CO 3 , into the nitrogen, and quickly added 0.556mmolCuI. Weigh 0.005mol of 1-chloroanthraquinone, add 30mL of N,N-dimethylformamide to dissolve (if there is a small amount of insoluble solid, use ultrasonic vibration until it becomes yellow and transparent), and quickly add it to the dropping funnel, start stirring and Slowly add dropwise (dropping is completed in about 10 minutes), start timing when the temperature rises to 100°C, and reflux for 12 hours.
[0024] (2) After the reaction, the reaction solution was naturally cooled to 35°C, water and dichloromethane (volume ratio: 2:1) were added, and neutral silica gel was used as a filter aid to filter under pressure to remove the mixed solution. The black flocculent precipitate was then subjected to operations such as extractio...
Embodiment 2
[0034] This embodiment provides a supercritical CO 2 Use the synthetic method of anthraquinone type active disperse dye precursor, other steps of this method are the same as embodiment 1. Wherein the reaction medium in the step (1) is 30 mL dimethyl sulfoxide; the yield of the dye precursor in the step (2) is 37.03%.
Embodiment 3
[0036] This embodiment provides a supercritical CO 2 Use the synthetic method of anthraquinone type active disperse dye precursor, other steps of this method are the same as embodiment 1. Wherein in step (1), 2,5-diaminotoluene sulfate and K 2 CO 3 The dosages are respectively 0.0075 mol and 0.015 mol, the reaction temperature is 110° C., and the reaction time is 6 h; the yield of the dye precursor in the step (2) is 38.79%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com