Electrolyte Formulation For Reduced Gassing Wide Temperature Range Cycling
A technology of non-aqueous electrolyte and electrolyte solution, applied in non-aqueous electrolyte storage battery, electrolyte storage battery manufacturing, lithium storage battery and other directions, can solve the problems of detrimental battery performance and life, increasing volume, weight complexity and cost, limiting battery application, etc. Achieve the effect of improving power, improving battery charge-discharge cycle efficiency, and reducing gas evolution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0125] Example 1: Electrolyte Formulation
[0126] Example electrolyte formulations according to the present disclosure include: LiPF 6 , 1.0M; LiTFSI, 0.15M; EC, 40vol.%; EMC, 45vol.%; DEC, 10vol.%; PC, 5vol.%; ES, 1.5wt.%; VC, 1wt.%; wt.%.
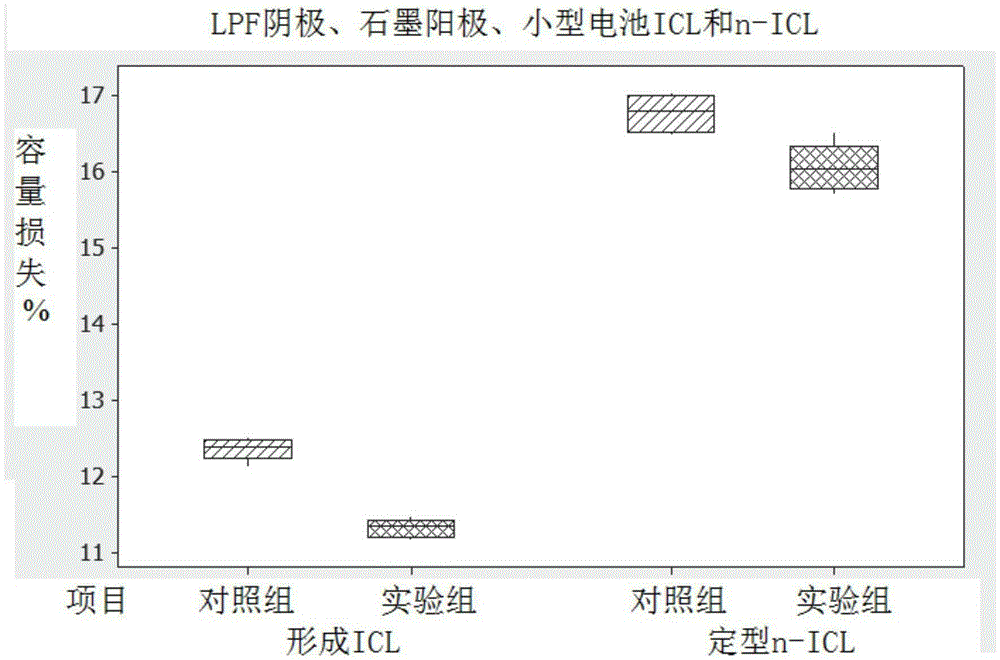
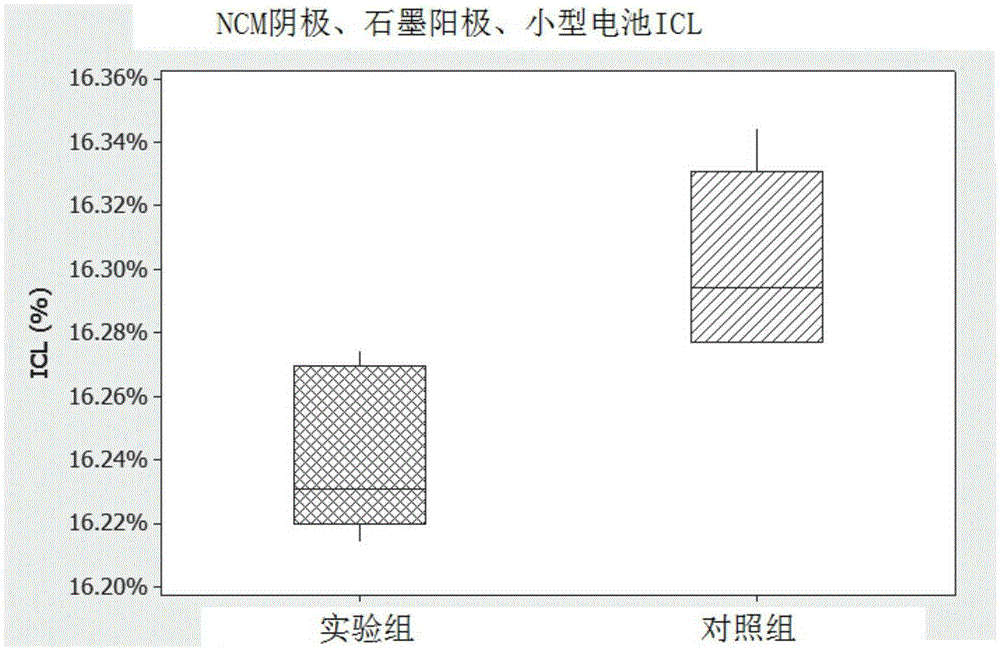
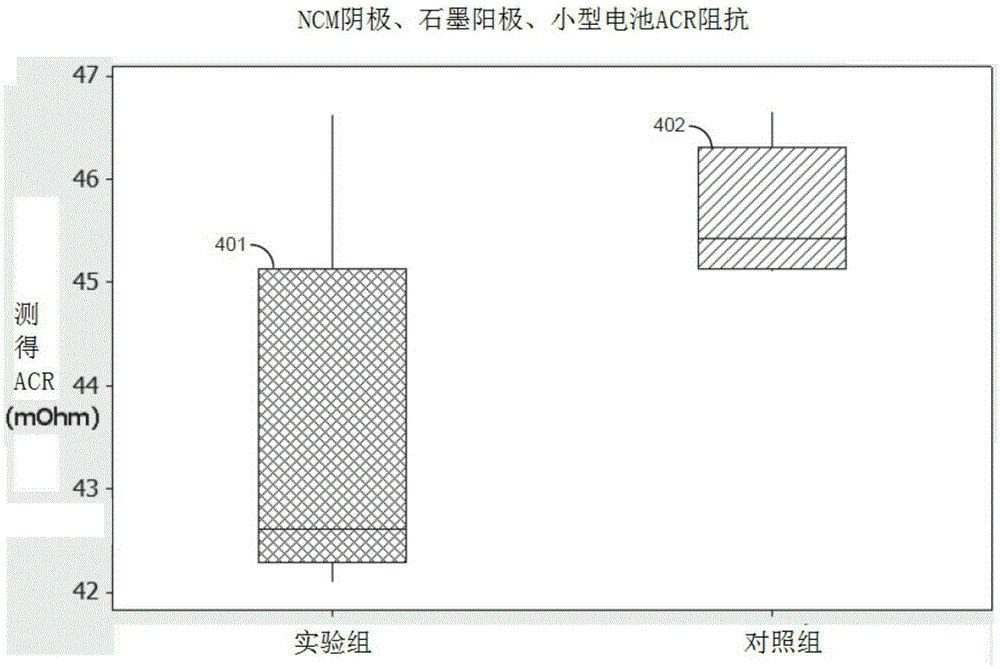
[0127] The electrolyte formulations were compared with the control electrolyte formulations, as follows and with reference to Figure 1 to Figure 10 discussed. The electrolyte formulation exhibits improved performance in low and high temperature tests relative to a control electrolyte formulation.
[0128]The first control electrolyte formulation contains: LiPF 6 EC, 30 vol.%; EMC, 55 vol.%; DEC, 10 vol.%; PC, 5 vol.%; ES, 1 wt.%; and VC, 2 wt.%. The first control electrolyte formulation contained the sulfonyl-containing first additive ES, but did not provide the salt solution and anti-gassing additives of the present application.
[0129] The second control electrolyte formulation contains: LiPF 6 , 1.15M; EC, 35vol.%; EMC, 40v...
example 2
[0148] Example 2: Electrolyte formulation for control group
[0149] The electrolyte formula of the control group was composed of 1MLiPF 6 Composition, wherein: EC:PC:EMC:DEC=35:5:50:10v / v%+VC2wt.%, "EC" means ethylene carbonate; "PC" means propylene carbonate; "EMC" means methyl ethyl carbonate ester; "DEC" means diethyl carbonate; and "VC" means vinylene carbonate.
example 3
[0150] Example 3: Electrolyte Formulation Containing ES Only
[0151] This electrolyte formula consists of 1MLiPF 6 Composition, wherein, EC:PC:EMC:DEC=35:5:50:10v / v%)+ES1wt%. Here, "ES" means vinyl sulfite. The addition of ES reduces the impedance because ES reacts with the anode to form a solid electrolyte interface (SEI) that is more ionically conductive than the control electrolyte mentioned above. However, during formation, cells with this electrolyte (ie, one containing only ES additives) cannot be charged because the SEI is unstable and a large amount of gas is generated during decomposition.
[0152] Figure 13 This effect is shown where a carbon-based anode is charged with a lithium half-cell for the first time (the "forming" phase of the SEI curve). A slurry containing: 92 wt% artificial graphite dissolved in N-methylpyrrolidone (NMP), 4 wt% conductive graphite additive and 4 wt% polyvinylidene fluoride ( PVDF) binder, dried in an oven and calendered to form t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Conductivity | aaaaa | aaaaa |
| Specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com