A non-canonical secretory protein and its application in protein secretory expression
A technology of secreting protein and secreting expression, which is applied in the field of genetic engineering and can solve problems such as cytoplasmic protein leakage
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
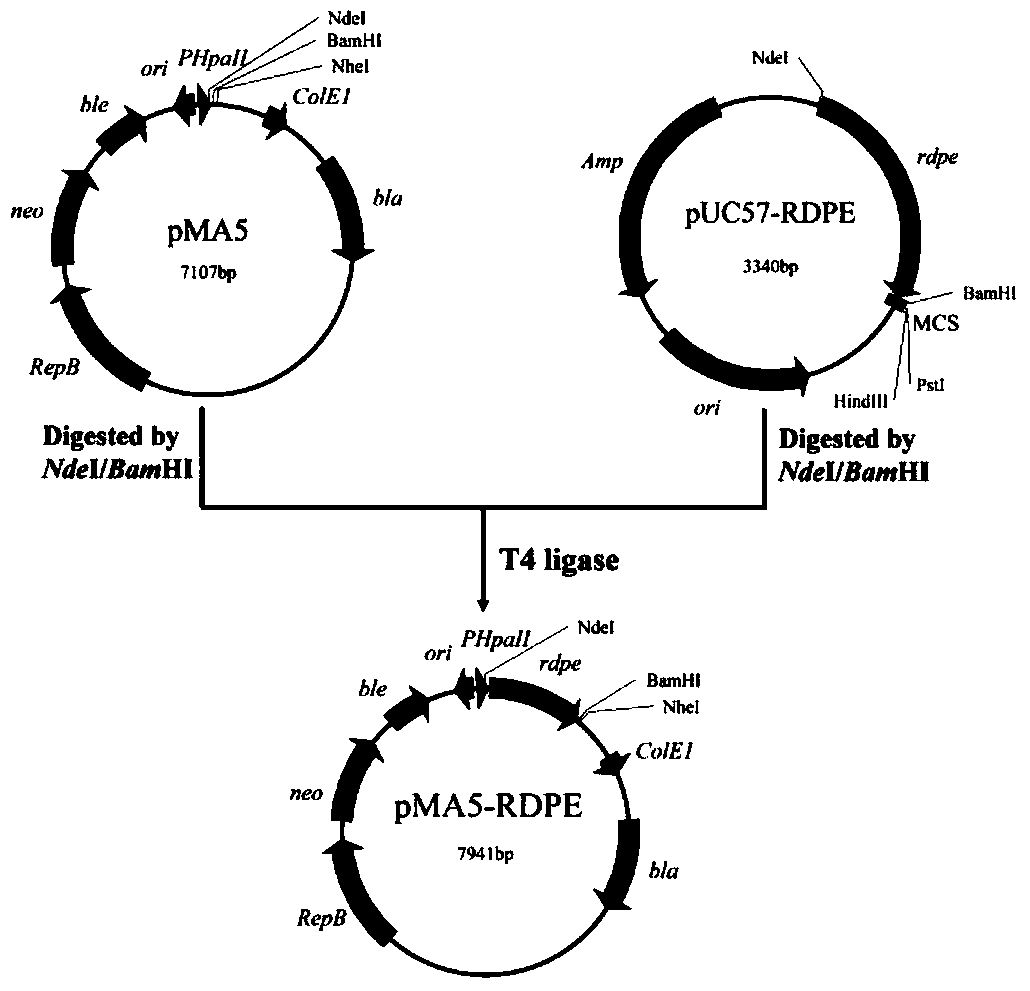
[0034] Example 1 Secretion and expression of RDPE in Bacillus subtilis
[0035] According to the source of rumen bacteria ( Ruminococcus sp. 5_1_39B_FAA) DPEase encoding gene rdpe The whole gene synthesis is carried out, and the 5' and 3' ends of the nucleotide sequence of the gene are respectively introduced Nde I and Bam HI restriction enzyme cutting site to facilitate the subsequent construction of expression vectors. The synthesized gene was connected to the pUC57 vector and named pUC57-RDPE.
[0036] Plasmids pUC57-RDPE and pMA5 were carried out separately Nde I and Bam HI double enzyme digestion treatment; the reaction condition of double enzyme digestion is 37°C water bath for 3 h. The double-digested products were recovered by gel respectively to obtain Nde I and Bam HI restriction site rdpe The fragment and the linearized pMA5 vector were ligated with T4 ligase for 30 min at room temperature; the ligation product was transformed into colony competen...
Embodiment 2
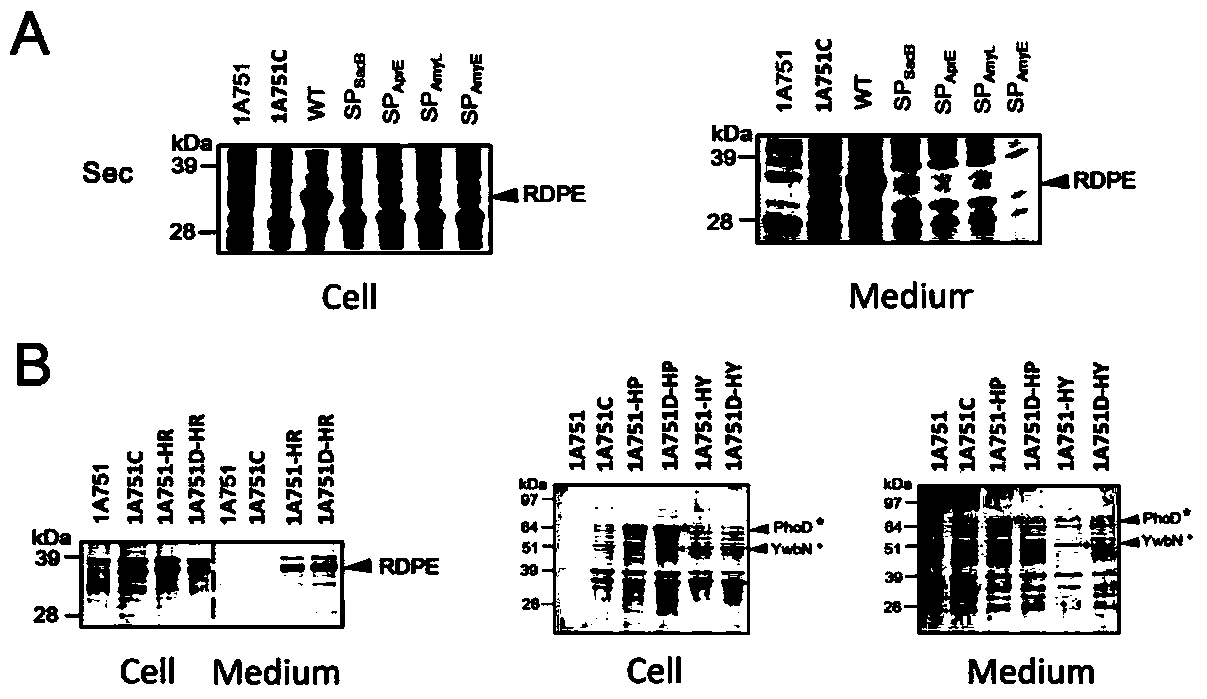
[0039] Example 2 SignalP predicts the signal peptide or signal sequence of RDPE
[0040] Using the signal peptide prediction software SignalP 4.1 (http: / / www.cbs.dtu.dk / services / SignalP / ) to analyze the amino acid sequence of RDPE, it was found that there is no typical signal peptide at the N-terminal of RDPE, nor in other regions. Any secretion signal sequence is present.
Embodiment 3
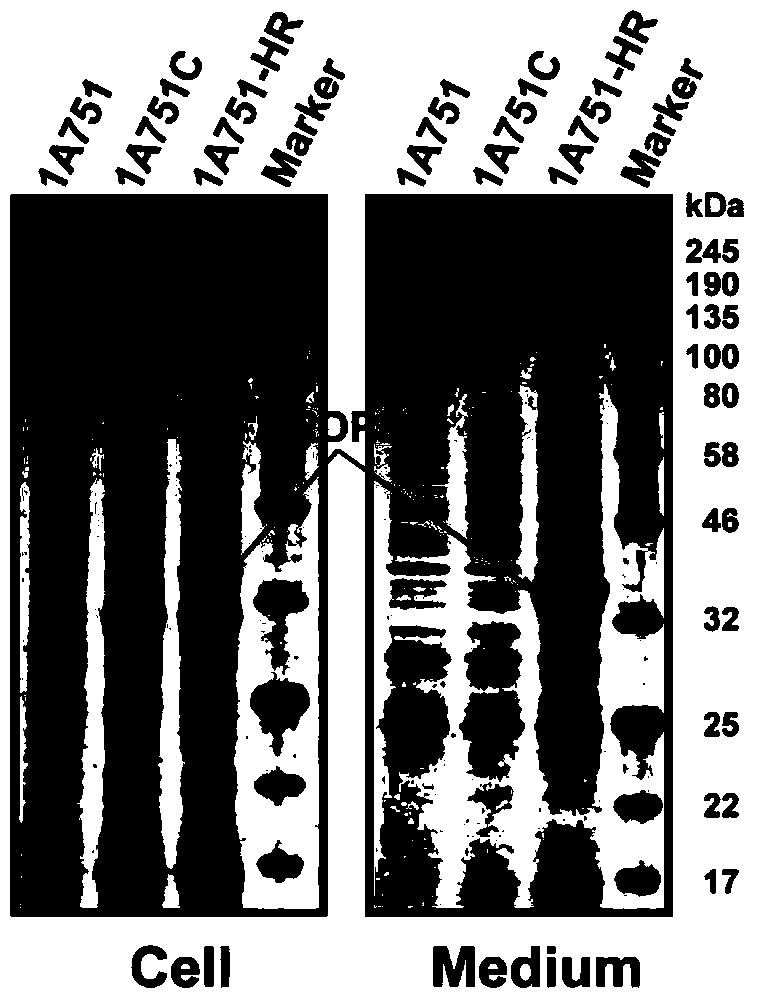
[0041] Example 3 RDPE cannot be secreted through the Sec and Tat secretion pathways
[0042] The partial recombinant plasmid construction method is based on the method in the literature (You et al. Appl EnvironMicrobiol 2012, 78:1593-5). The four Sec signal peptides (SP sacB 、SP AprE 、SP AmyL and SP AmyE , The nucleic acid sequences are respectively shown in SEQ ID NO. 2, 3, 4 and 5), and the PCR products obtained by cloning were respectively gel-recovered. Using pMA5-RDPE as a template, primers were used to amplify the linearized vector pMA5-RDPE, and the resulting PCR product was gel-recovered. The above four signal peptide fragments and the linearized vector pMA5-RDPE were subjected to POE-PCR (Prolonged overlap extension PCR), and the obtained fusion products were directly transformed into colon competent cells DH5α, respectively, to obtain recombinant expression plasmids pMA5-SP sacB -RDPE, pMA5-SP AprE -RDPE, pMA5-SP AmyL -RDPE and pMA5-SP AmyE -RDPE. The constr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com